Journal of Osteoporosis and Physical Activity
Open Access
ISSN: 2329-9509
ISSN: 2329-9509
Research Article - (2020)Volume 8, Issue 4
The aim of the study was to determine the effect of a dedicated exercise program on important menopausal risk factors and complaints in osteopenic early-postmenopausal women. Fifty-four women, 1-5 years postmenopause with osteopenia were randomly assigned (a) to a high impact weight bearing/high intensity, high velocity resistance training group (EG: n=27) exercising three times a week or (b) to an attention control group (CG: n=27). Study endpoints were body composition including Bone Mineral Density (BMD) at the Lumbar Spine (LS) as determined by Dual-Energy X-Ray Absorptiometry (DXA), menopausal symptoms, low back pain, lower extremity strength and power. After 28 weeks of intervention, significant effects were determined for free fat mass (EG: 0.48±0.68 kg vs CG: -0.15±0.88 kg, standardized mean differences (SMD): 0.80, p=.005), total body fat mass (EG: -1.19±1.26 kg vs CG: 0.36±1.59 kg,SMD: 1.08, p=.001), abdominal body fat rate (-1.26±1.99% vs 0.54± 1.53%, SMD: 1.02, p=.001), low back pain frequency (SMD: 0.55, p=.049) and severity (SMS: 0.66, p=.018), lower extremity strength (SMD: 1.46, p<.001) and jumping height (SMD: 0.92, p<.001) in the EG compared with the CG. Menopausal complaints improved in both groups, but changes were only significant in the EG (SMD: 0.33, p=.232). We did not determine significant exercise effects on LS-BMD (SMD: 0.26, p=.351). In conclusion, we demonstrate the general effectiveness of a multipurpose exercise protocol on various risk factors and complaints related to the menopausal transition. Future assessments have to determine the exercise effects on BMD, possibly the most challenging physiologic outcome of this ongoing project.
Exercise; Menopausal transition; Early-postmenopausal; Body-composition; Menopausal symptoms; Resistance training
Clinical Trials.gov: NCT03959995
The menopausal transition is a crucial phase in women’s life. Apart from psychosocial effects, a range of physiologic systems are affected, particularly by the pronounced decline of estradiol (E2), the most potent member of the estrogen family [1-5]. Clinical manifestations of the menopausal transition include changes in body composition and fat distribution, accelerated bone loss, functional declines and menopausal symptoms [2,6-10]. With respect to bone, estrogen (E2) deficiency leads to increased bone turnover and subsequent bone resorption. The (early-) postmenopausal bone loss can be thus referred predominately to the lack of E2 [3,4]. Exercise may be the most promising non-pharmaceutic strategy to offset some of these negative consequences [11-14]. However, it is difficult to design exercise protocols that simultaneously improve menopausal symptoms, physical fitness, cardiometabolic and musculoskeletal risk factors [12,14-16]. This need for compact, multi-purpose exercise programs also becomes obvious when considering that only the minority of (early-) postmenopausal women might be able or motivated to exercise very frequently in order to address each desired outcome by dedicated exercise programs [17,18]. In the present ACTLIFE-ER study (Physical ACTivity: The tool to improve the quality of LIFE in osteoporosis people-Erlangen Project), we aimed to determine the effect of a multipurpose exercise program on risk factors and complaints of early-postmenopausal women with osteopenia or osteoporosis.
Our primary hypothesis was that the Exercise Group (EG) of early postmenopausal women with osteopenia and osteoporosis demonstrated significantly higher effects on (a) free fat mass compared with a corresponding Control Group (CG). Core secondary hypotheses were that the EG demonstrated significantly higher effects on (b) total and (c) abdominal body fat compared with a corresponding CG.
Further secondary hypotheses were that the EG demonstrated significantly higher effects on (d) maximum leg extension strength and (e) power compared with a corresponding CG. Further, we hypothesize that changes of (f) menopausal complaints and (g) low back pain in the EG will be more favorable compared with the CG.
Finally, our experimental hypothesis was that after 28 weeks of exercise no significant group differences (EG vs CG) for (h) BMDchanges at the lumbar spine Region of Interest (ROI) could be observed.
The ACTLIFE-ER (Erlangen) study was an 18-month randomized, controlled, semi-blinded exercise trial in a parallel group design with one exercise and one attention control group. ACTlIFE-ER is part of the ACTLIFE-project, a European Project focusing on the development and dissemination of validated best practice exercise on the secondary and tertiary prevention of osteoporosis. In particular, the project addresses Bone Mineral Density (BMD) and fear of falling in people with osteopenia and osteoporosis. While the latter part of the project was conducted in Bologna, Italy, as part of this project, ACTLIFE-ER examined on the effect of a dedicated exercise protocol to address menopausal risk factors under special regard of the early menopausal bone loss. The Institute of Medical Physics (IMP), University of Erlangen-Nürnberg (FAU), Germany is the responsible partner for the project, which has been approved by the FAU Ethics Committee (number 118_18b) and the Federal Bureau of Radiation Protection (BfS, number Z5-22462/2-2018- 055). The project fully complies with the Helsinki Declaration [19]. After receiving detailed information, all the study participants gave their written informed consent. Project registration was conducted under ClinicalTrials.gov: NCT03959995. The present publication focuses on body composition, menopausal complaints and physical fitness parameters during the first 28 weeks of the intervention (February 2019-September 2019).
Participants
Using citizen registers provided by the municipal registry office, 2500 randomly selected women 48-60 years living independently in the area of Erlangen-Nürnberg, Germany were contacted by personalized letters, which already included the most important eligibility criteria (i.e. menopausal and exercise status, medication). 332 women expressed an interest and were subsequently assessed for eligibility by phone calls, structured interviews and after general eligibility finally by bone densitometry. Inclusion criteria applied were (a) early-menopause (i.e. 12-60 months amenorrhea (b) osteopenia or osteoporosis (T-Score -1 to -4 SD1) at lumbar spine, femoral neck or total hip region of interest (ROI). We excluded women who reported (a) secondary osteoporosis or osteoporotic fractures, (b) medication2 and diseases3 known toaffect bone metabolism or prevent group exercise, (c) acute or recent history of cancer (last 5 years), (d) any type of high impact or resistance exercise (>45 min/week)4 during the last 5 years, (e) regular “high” alcohol consumption (i.e.≥60 g/d on 5 days/week), (f) absence for more than 6 weeks during the intervention period. Seventyfive of the ninety-two eligible women accepted the invitation to information meetings. After detailed study information, 21 women quit the study due to the lack of option to join their preferred group (i.e. exercise or control group). Thus, 54 women were included and willing to participate. Figure 1 illustrates the recruitment process and participant flow through the study.
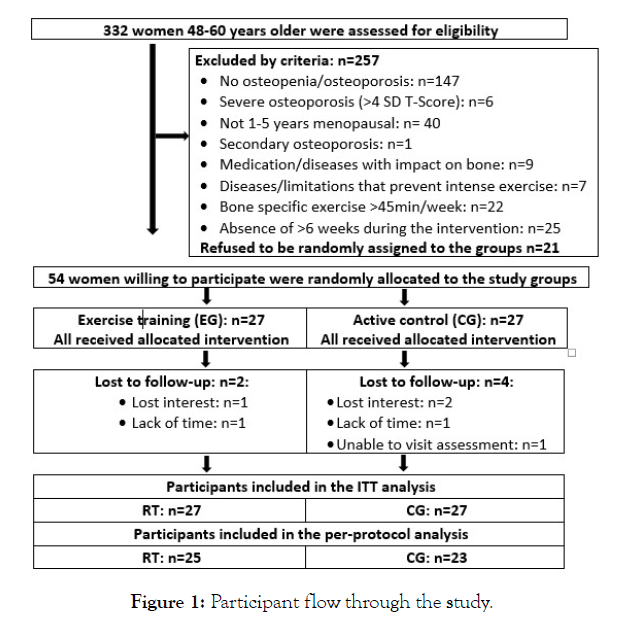
Figure 1: Participant flow through the study.
Randomization procedures
Participants were stratified for baseline lumbar spine BMD (2 strata) and randomly and balanced assigned to the study arms. Participants allocated themselves to the exercise or control group by drawing lots from small opaque capsules (“kinder egg”, Ferrero, Italy) and drawn from a bowl. A researcher not involved in the present project prepared the lots and supervised the randomization procedure. Of importance, neither researchers nor participants knew the allocation beforehand. After the randomization procedure, the researcher responsible (MH) enrolled participants and instructed them in detail about their study status, corresponding dos and don’ts and fixed training dates with the participants.
Blinding
Outcome assessors and test assistants were kept unaware of the participants' group status (EG or CG) and were not allowed to ask, either. Although we implemented an active control, we did not attempt to blind participants about group status.
Study procedure
ACTLIFE-ER focused on the effects of exercise on menopausal risk factors and complaints with special regard to BMD. All participants were provided with Cholecalciferol (Vit-D) and Calcium (Ca) supplements in order to meet present recommended intake (i.e. 800 IU/d Vit-D, 1000 mg/d Ca) [20]. Participants were asked to maintain their dietary routines during the study, further, all women were requested to maintain usual physical activity and exercise habits in addition to any exercise undertaken in the present intervention.
Intervention
Exercise group: The ACTLIFE-Erlangen study applied a blockperiodized exercise training protocol with high intensity phases over 10-12 weeks interspersed with 4 weeks of recreational exercise between each phase. During the linearly periodized5 high intensity phases, we scheduled three supervised sessions/week in our lab and a well-equipped gym. During the recreational phases, two supervised sessions and one video-guided home exercise session (15 min) were prescribed (Figure 2). Participants were provided with detailed training logs that prescribed exercises, number of repetitions (reps), movement velocity and absolute exercise intensity (or “effort”) (see below).
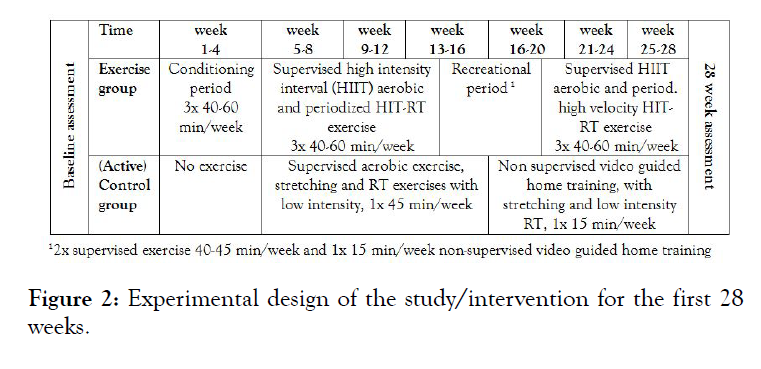
Figure 2: Experimental design of the study/intervention for the first 28 weeks.
During the first four weeks of the study, we focused on briefing, familiarization, learning of proper lifting technique and rating of perceived exertion. Starting with phase 1, participants completed a 40-45 min session of weight bearing and strength training twice a week (Monday and Wednesday) in our lab, predominately without dedicated resistance exercise machines. A 5 min warm up was followed by 15 min of progressively increased high intensity interval training (HIIT) including high impact aerobic dance and movements with ground reaction forces (GRF) of 2.5-3x body weight. On Mondays, 60 sec of high intensity phases (≈80-85% HRmax) were intermitted by 60 sec of lower intensity (≈65-70% HRmax), on Wednesdays we scheduled a 30 sec/30 sec protocol. During the Dynamic Resistance Training (DRT) sequence, a single set approach that addressed all the main muscle groups by 12 exercises/session (calf rises, lunges, leg-press, half squat, (half) squats, back extension (roman chair), deadlifts, single side lateral rows, trapezius and latissimus pulldowns, bench dips, incline dumbbell bench press) was applied in a circuit mode. Loading phases versus rest periods varied between 40/30 sec, 60/30 sec and 80/30 sec. Applying a time under tension (TUT) of 2 (concentric)-1 s (isometric)-2 s (eccentric), the number of repetitions (reps) averaged between 8-16 reps.
During the second high-intensity DRT phase, we manipulated movement velocity. Applying a TUT that varied between explosive movement6-1 s (isometric) 1 s/isometric)-2 s (eccentric) and 4 s-0 s-4 s, the number of repetitions (reps) varied between 5 and 20 reps per session. Similar to phase 1, exercise intensity was prescribed using the Repetition In Reserve (RIR) approach of Zourdos et al.and the set endpoint definition of Steele et al. [21,22]. So far (28 weeks), exercise intensity per set was prescribed to incomplete work to failure (nRM, eg repetition maximum minus 1-2 reps)7. Total duration of the exercise sessions in our lab was consistently maintained at ≈45 min.
On Fridays or Saturdays, participants trained on dedicated resistance training machines in a well-equipped gym. After careful briefing and instruction, the women were free to visit the gym between 13:00 and 16:00. They completed a 15min warm up on a cross-trainer (65-70% HRmax), before starting the DRT. The supervised single set exercise approach of the gym training addressed 13-15 exercises for all main muscle groups (leg press, -extension, -curls, -adduction, -abduction, latissimus front pulleys, rowing, roman chair, trunk extension, -flexion, inverse fly, bench press, military press, lateral raises, shoulder/triceps press). Parallel to the circuit training, we scheduled a linearly periodized exercise protocol with a varying number of repetitions, movement velocity, and varying intensity (nRM: Maximum effort minus 1-2 reps)8. Rest pause between the sets averaged 60 sec-120 sec, total length of a session averaged 60-70 min.
During the 4-week recreational period, one circuit session (see above), one 45 min session of stretching and floor exercises (see control group) with low intensity and effort, and one video guided home training session (see control group) of 15 min were conducted.
Control group
During the 18month intervention period, 3 cycles of 12 weeks of supervised group exercises (45 min) intermitted by 12-14 weeks of non-supervised, video-guided home training (15 min) were scheduled for the control group (Figure 2).
The supervised training session consisted of 15 min of walking/ marching exercise, 20 min of stretching and easy floor exercises and 10 min of cool down. One set of stretching routines with 30 sec/ exercise and moderate intensity9 addressed muscle groups in the lower and upper calf, hamstring, thigh, gluteal, hip flexors, lower and upper back, abdominal, and pectoralis sites. Floor exercises in a sitting, supine or prone lying position predominately included isometric exercises for trunk muscle groups. Two sets each of 6-8 varying isometric exercises/session with 10 sec of moderate intensity (“5” on Borg CR 10) and 30 sec of rest were conducted [24]. During the 10 min of cool down, the instructor presented different “fantasy journeys” to encourage general relaxation or body awareness. The first supervised 12-week period started in March 2019 and ended in June 2019.
During the non-supervised phases, participants were provided with training videos that summarized the joint training session. Fifteen minutes of stretching and isometric exercises, which had been demonstrated during the supervised training period, were included Participants were asked to undertake this training on Fridays and record their participation in their training logs. The non-supervised training period finished immediately before the 28- week follow-up assessment.
Vitamin-D and Calcium supplementation
Independently of the baseline 25-OH-D serum concentration, participants were requested to take two capsules of cholecalciferol (MYPROTEIN, Cheshire, UK) of 2,500 IE/d once a week (i.e. 5,000 IE/week). As per German guidelines, we aimed to ensure a calcium intake of 1,000 mg/d for all the participants [20]. The amount of dairy dietary calcium was evaluated using dietary calcium questionnaires (Rheumaliga, Switzerland). The required calcium was provided by calcium capsules (Sankt Bernhard, Bad Dietzenbach, and Germany), with one capsule containing 250 mg of calcium carbonate.
Compliance with the exercise intervention
Participants signed an attendance list for each training session. Further, the gym's chip card system allowed accurate assessment of participant attendance rate and exercise duration during the gym session. Nevertheless, the participants' training logs were checked for attendance after each of the meso-cycles. In parallel, instructors checked participant compliance by monitoring the load/repetition proportion during the sessions. Finally, the principal investigator checked the training logs of the EG participants, particularly to determine compliance with the exercise (intensity) prescription.
Study outcomes
Primary study outcome
• Fat free mass changes as determined by Dual-Energy X-Ray Absorptiometry (DXA) from baseline to 28-week follow-up assessment.
Secondary study outcomes
• Total body fat changes as determined by DXA from baseline to 28-week follow-up assessment.
• Abdominal body fat changes as determined by DXA from baseline to 28-week follow-up assessment.
• Changes of menopausal symptoms as determined by menopausal rating scale II from baseline to 28-week followup assessment [25].
• Maximum dynamic hip-/leg-extension strength changes as determined by an isokinetic leg press from baseline to 28- week follow-up assessment.
• Maximum jumping height as determined by a force plate from baseline to 28-week follow-up assessment.
• Back pain severity and frequency at the lumbar spine site as determined by questionnaire from baseline to 28-week follow-up assessment.
Experimental study outcome
• BMD changes at the lumbar spine as determined by Dual-Energy Absorptiometry (DXA) total body scan from baseline to 28-week follow-up assessment.
Changes of trial outcomes after trial commencement
No changes of trial outcomes were made after trial commencement.
Assessments
The 28-week assessments were conducted during the two first weeks of a 4-week regeneration period. Participants were asked to maintain their habitual physical activities and dietary habits but not to exercise 48 h prior to the tests. All the tests/assessments were consistently conducted and analyzed by the same research assistant. Further assessments were performed at the same time of the day (±90 min) at the same location, with exactly the same calibrated devices and in identical order.
We determined body height using a Holtain stadiometer (Crymych Dyfed., Great Britain) and used direct-segmental, multi-frequency Bio-Impedance-Analysis (DSM-BIA, InBody 770, Seoul, Korea) to determine body mass and body composition10. Body composition and BMD at the lumbar spine, proximal femur ROIs and total body was evaluated by DXA (QDR 4500a, Discovery-upgrade, Hologic Inc., Bedford, USA). Abdominal body fat was segmented between the lower end of the 12th thoracic vertebra and the upper end of the iliac crest. Of importance, at 7 month follow-up we conducted only a total body DXA-scan. Segmentation of LS-BMD and abdominal body fat was conducted using the “compare mode”, so that area and placement of the baseline assessment could be exactly reproduced.
Maximum isokinetic leg-/hip-extensor strength was measured with an isokinetic leg press (CON-TREX LP, Physiomed, Laipersdorf, Germany). The test was conducted in a sitting, slightly supine position, with fixation by hip and chest straps. The participants' feet were positioned on a flexible sliding footplate and also fixed with straps. The range of motion during leg extension was 30º to 90º within the knee angle, velocity of the movement was 0.2 m/s. After detailed briefing and familiarization with the testing procedure, the women performed five reps with maximum effort (“push as strongly as possible”). Participants completed two trials with two minutes of rest between trials. We included the higher value of both trials in the data analysis.
Lower extremity power was determined by Counter Movement Jump (CMJ) with hands on hips (i.e. no arm swing) during the test. Participants were asked to “jump as high as possible” with an explosive movement starting from an upright position. We did not limit countermovement depth, however, we required participants to maintain extension in the hip, knee, and ankle joints to prevent any additional flight time by bending their legs. A force platform (KMP Newton GmbH, Stein, Germany) was used to determine jumping height (present outcome) and power. Jumping height was calculated automatically by the software provided by the manufacturer based on ground reaction forces.
Participants completed a standardized questionnaire at baseline that asked for (a) demographic parameters, (b) diseases, pharmacologic therapy, dietary supplements and operations with particular regard for osteoporosis risk and participation in an intense exercise study, (c) physical limitations, (d) falls and injurious falls, (e) injuries and low trauma fractures within the last year, (f) pain frequency and severity at the lumbar spine region, (g) lifestyle, including physical activity and exercise and (h) menopausal complaints using the Menopause Rating Scale (MRS II) provided by Hauser et al. [25- 28]. After 28 weeks, all participants conducted a follow-up (FU) questionnaire. Apart from general pain frequency and severity and menopausal complaints (MRS II), the FU questionnaire focused on changes might affect our study endpoints. Questionnaires were carefully checked for consistency, completeness and accuracy in close interaction between the primary investigator and participants.
All the participants were asked to conduct four-day diet records at BL and after 28-weeks Participants were carefully briefed and instructed on how to keep the diet records (Freiburger Nutrition Record (nutri-science, Hausach, Germany)). The Freiburger Nutrition Record based on a tally-list of how often the food products were consumed. Participants were asked to protocol 3 weekdays and one weekend day representative for their nutritional habits. Results of the diet records were carefully analyzed by the same researcher and discussed with the participants. In cases of unlikely results, (e.g. energy intake <1000 kcal/d or >3500 kcal/d), the women were requested to provide another diet record based on more representative days.
Sample size calculation
The sample size calculation was based on the primary study outcome of the ACTLIFE-ER project BMD-changes at the LS after 18 months. Assuming an effect (Δ-EG vs Δ-CG) on BMD-LS of 2.0±2.5% determined in comparable studies and applying a t-test based sample size calculation, the required sample size to generate a 80% power (1-β) and alpha=.05 is 25 participants per group11 [29,30]. Considering that we focus on FFM changes at 28week FU, the 27 participants/group generate a power of 86% (alpha=.05) for detecting a realistic and meaningful difference of 500±600 g between the groups.
Statistical analysis
As prescribed for an RCT, we conducted an intention to treat (ITT) analysis that included all participants randomly assigned to the two study arms (EG vs CG). Additionally, a per-protocol analysis was performed that included only participants with complete datasets independently of compliance or other confounding aspects. Multiple imputations (ITT) were calculated using R statistics software in combination with Amelia II [31,32]. The full data set was used for multiple imputations, imputation being repeated 100 times. Imputation for primary and secondary outcomes worked well, as confirmed by over imputation diagnostic plots provided by Amelia II. The application of statistical (Shapiro-Wilks) and graphical (qq-plots) procedures confirmed the normal distribution of the study endpoints addressed. To identify group differences, pairwise t-test comparisons (EG vs CG) with pooled SD were applied. Alternatively, a repeated measure ANOVA (group by time interaction) was calculated within the per-protocol analysis. We consistently applied 2-tailed tests, significance was accepted at p<0.05. We also calculated effect sizes (Standardized Mean Difference: SMD) according to Cohen (Cohens d) [33].
Table 1 displays baseline characteristics of the ACTLIFE-ER study. Most baseline characteristics were evenly distributed, only 25 OHD concentration and alcohol intake varied, albeit non-significantly. Protein intake was relatively (p=.760) high in both groups (EG: 1.20±0.21 vs 1.18±0.27 g/kg body mass/d). Dietary calcium intake was similarly low in both groups, in contrast to cholecalciferol (n=8 in EG and CG respectively), none of the women used calcium supplements (Table 1). So far, no adverse effects of the intervention have been reported or monitored.
| Variable | EG (n=27) MV ± SD |
KG (n=27) MV ± SD |
p |
|---|---|---|---|
| Age [years] | 53.6 ± 2.0 | 54.5 ± 1.6 | .441 |
| Body height [cm] | 164.2 ± 6.0 | 164.5 ± 8.2 | .889 |
| Body mass [kg] | 64.0 ± 9.6 | 67.4 ± 14.6 | .320 |
| Calcium intake [mg/d] | 645 ± 252 | 642 ± 265 | .972 |
| Vit-D level (25-OHD) [ng/ml] | 27.8 ± 11.7 | 21.6 ± 10.8 | .051 |
| Age at menarche [years] | 13.6 ± 1.6 | 13.7 ± 1.7 | .838 |
| Age at menopause [years] | 49.8 ± 3.8 | 51.0 ± 3.0 | .189 |
| Exercise volume [min/week] | 63.7 ± 47.5 | 45.6 ± 38.4 | .128 |
| Waist circumference [cm] | 87.8 ± 8.6 | 91.1 ± 9.9 | .191 |
| Energy intake1 [kcal/d] | 2009 ± 444 | 2067 ± 355 | .613 |
| Protein intake1 [g/d] | 75.8 ± 14.4 | 78.0 ± 14.7 | .597 |
| Carbohydrate intake1 [g/d] | 223 ± 69 | 227 ± 57 | .841 |
| Fat intake1 [g/d] | 84.1 ± 20.6 | 85.7 ± 23.8 | .806 |
| Alcohol intake1 [g/d] | 2.63 ± 4.06 | 5.53 ± 6.39 | .066 |
| Ovariectomy <50 years [n] | 12 | 0 | .313 |
| Family disposition3 [n] | 7 | 9 | .551 |
1 n=51 (EG: n=26, CG: n=25); 2 at age 47 years; 3 verified osteoporosis in close relatives (parents, aunts, uncles, grandparents)
Table 1: Baseline characteristics of the ACTLIFE-ER study.
Two women in the exercise and three women in the CG quit the study (Figure 1). Reasons for withdrawal were lack of time and loss of interest. The two CG women who cited loss of interest for their withdrawal claimed that the sessions were not intensive enough.
On average, participants of the EG attended 64±10 of 79 sessions (80±13%). The attendance rate of the CG for the 12 week supervised and 12week non-supervised exercise period was similar (79±15%). In summary, compliance with the training protocol was satisfactory, however, based on enquiries during the sessions and retrospective analysis of the training logs, we speculate that about one quarter of the exercises were conducted with lower than recommended effort. This particularly refers to the first high intensity phase.
Primary and secondary study outcomes
Based on comparable baseline data, FFM increased significantly in the EG (p<.002) and was maintained in the CG (p=.352). Differences between the groups were significant (p<0.005, SMD: 0.80) (Table 2).
| CG MV ± SD |
EG MV ± SD |
Difference MV (95% CI) |
p-value | |
|---|---|---|---|---|
| Fat Free Mass (FFM)[kg] | ||||
| Baseline | 41.34 ± 6.43 | 40.39 ± 4.78 | ------------ | .544 |
| Changes | -0.15 ± 0.88 | 0.48 ± 0.68** | 0.63 (0.20 to 1.06) | .005 |
| Total Body Fat [%] | ||||
| Baseline | 34.2 ± 6.9 | 34.0 ± 5.0 | ----------- | .866 |
| Changes | 0.36 ± 1.59 | -1.19 ± 1.26*** | 1.55 (0.76 to 2.33) | <.001 |
| Abdominal Body Fat [%] | ||||
| Baseline | 28.6 ± 9.2 | 28.5 ± 7.0 | ------------ | .972 |
| Changes | 0.54 ± 1.53 | -1.26 ± 1.99*** | 1.80 (1.11 to 2.64) | <.001 |
** p<.01; *** p<.001
Table 2: Baseline data and changes of anthropometric parameters in the CG and EG.
In parallel, total body fat mass (p<.001) and abdominal body fat rate (p=.001) decreased significantly in the EG and increased in the CG (total: p=.207, abdominal body fat: p=.119). Differences between EG and CG for total (p<0.001, SMD: 1.08) and abdominal body fat (p<.001, SMD: 1.02) were significant (Table 2).
Menopausal symptoms as determined by MRS II improved in both groups, however, the changes from pre to post-intervention were only significant in the EG (EG: p=.026 vs CG: p=.566) (Table 3). No significant between group difference for MRS was observed (p=.232, SMD: 0.33). The same result of non-significant differences between EG and CG (p≥.225, SMD≤0.35) was observed for subscales (“dimensions”) of MRS II, i.e. somato-vegetative, psychological, and urogenital complexes of symptoms (not given).
| CG MV ± SD |
EG MV ± SD |
Difference MV (95% CI) |
p-value | |
|---|---|---|---|---|
| Menopause Rating Scale II [score points]a | ||||
| Baseline | 1.20 ± 0.52 | 1.06 ± 0.64 | -------------- | .365 |
| Changes | -0.06 ± 0.54 | -0.22 ± 0.44* | -0.16 (0.11 to -0.43) | 0.232 |
| Low back pain frequency [score points] b | ||||
| Baseline | 2.70 ± 2.28 | 2.37 ± 2.00 | -------------- | .365 |
| Changes | 0.09 ± 1.37 | -0.76 ± 1.69* | 0.85 (0.05 to 1.69) | .049 |
*p<.05; a Scale from 1 (no complaints) to 5 (very serious complaints); b 0 (no pain) to “7” (chronic pain)
Table 3: Data on menopausal symptoms and low back pain in the CG and EG.
Low back pain frequency and severity decreased significantly in the EG (p=.011 and p=.004) and was maintained in the CG (p=.769 and p=.582) (Table 3). Differences for pain frequency (SMD: 0.55, p=.049) and severity (SMD: 0.66, p=.018) were significant.
Maximum hip-/leg extension strength (p<.001)12 and power (p<.001) as determined by isokinetic leg-press and force plate increased significantly in the EG and improved slightly (strength: p=.606, power: p=.0.85) in the CG (Table 4). Differences between the groups were significantly higher for the EG (strength p<.001, SMD: 1.46 versus power: p=.002, SMD: 0.92).
| CG MV ± SD |
EG MV ± SD |
Difference MV (95% CI) |
p-value | |
|---|---|---|---|---|
| Maximum hip-/leg extension strength (leg press) [N] | ||||
| Baseline | 2056 ± 576 | 2073± 429 | -------------- | .901 |
| Changes | 27 ± 221 | 409 ± 298 *** | 382 (236 to 528) | <.001 |
| Maximum jumping height (counter movement jump) [cm] | ||||
| Baseline | 19.1 ± 3.2 | 19.4 ± 3.7 | -------------- | .807 |
| Changes | 0.78 ± 1.83 | 2.85 ± 2.62 *** | 2.07 (0.81 to 3.32) | .002 |
*** p<.001
Table 4: Baseline data and changes of maximum strength and power in the CG and EG.
Finally, based on comparable baseline areal BMD values in the EG (0.953±0.102 versus CG: 0.926±0.129 g/ cm2, p=.406), we did not observe any significant changes in the EG (0.004±0.028 g/ cm2, p=.472) or CG (-0.003±0.026 g/ cm2, p=.555) or differences from pre to post-intervention between the groups (p=0.351, SMD: 0.26) for LS-BMD.
Thus, apart from hypothesis (f) (i.e. effects on menopausal symptoms), all the hypothesis addressed were confirmed. In each case, the per-protocol analysis confirmed the result of the ITT, predominately with slightly higher effects.
We did not observe any relevant changes or between groups differences in parameters that might have affected our result. Exercise and physical activity outside the ACTLIFE-ER protocol was maintained in the EG and the CG. With respect to dietary intake, we observed only comparable minor increases of energy (EG: 2.8±7.0% vs CG 3.4±10.8%) and decreases in protein intake (2.2±7.0 g vs 1.9±6.3 g) in both groups. No participant reported diseases or changed pharmaceutic treatment with an impact on study outcome during the study period.
In this study, we determined the effect of a multipurpose exercise program on menopausal risk factors and symptoms in early postmenopausal women with osteopenia and osteoporosis. In summary, we observed significant positive effects on body composition, maximum leg strength and power and low back pain, albeit not on menopausal complaints and Bone Mineral Density at the LS. Notably, the latter is the primary study outcome of the ACTLIFE-ER project, therefore at first one might be surprised that we not only consider this outcome as a subordinated outcome at 7month FU but also expect non-significant effects on LSBMD changes. However, we are convinced that exercise-induced bone changes in adults were generated more by remodeling than by modeling [34]. Since cancellous bone remodeling takes about 200 days in normal bone, exercise studies ≤7 months of lengthdetermine the full amount of new mineralized bone [35]. Given the familiarization period of ACTLIF-ER, the magnitude and strain rate of mechanical stimuli might have been below the threshold for bone adaptation during the first 4-6 weeks [36,37]. Nevertheless, there are some exercise studies which observed positive effects on BMD-LS as early as after 6-7 months [38-42]. However, the BMD decline in the CG13 rather than a positive change in the EG may be responsible for the positive effect observed [38-41].
However, revisiting the primary study outcome of the 28week FU, we observed a significant positive effect on fat free mass that averaged 517 g (95% CI: 169-864 g). Net exercise effect on total and abdominal fat mass was also significant and verified with high effect sizes (SMD: 1.08 and 1.02). Only few exercise studies specifically focus on body composition changes during the menopausal transition and the early-postmenopausal years [43-46]. Applying a roughly similar exercise protocol14 for EG and CG, we also addressed exercise effects on menopausal risk factors in earlypostmenopausal (1-3 years post) Caucasian women in our earlier TRACE (TRAining and Cimicifuga racemosa Erlangen) study [29,47]. While DXA-technique widely confirmed our result of positive changes in FFM (535 g, 95% CI: 131-938 g) in the TRACEEG, we were unable to demonstrate relevant effects on total and abdominal body fat mass (p≥.703, SMD<0.2) in TRACE, although we applied a high proportion of endurance exercise. Consequently, we applied lower exercise volume but higher intensity during the HIIT-based endurance sequence, further, we placed emphasis on DRT with high relative intensity in ACTLIFE-ER. From a clinical perspective, we regard our results on muscle and fat mass as very positive. Apart from physical appearance, and physical fitness, exercise induced changes of body composition are inversely related to cardiovascular and cardiometabolic risk in postmenopausal women [48-50].
Corresponding to TRACE [29], we observed positive effects on menopausal symptoms as determined by the MRS II scale, that were however not significant [25]. In parallel, TRACE and ACTLIFE-ER did not determine significant exercise effects on different aspects of menopausal complaints, i.e. somato-vegetative, psychological, and urogenital dimensions, summarized in the MRS II. While there is some evidence that exercise positively affects psychological factors (e.g. wellbeing, anxiety, depression), a recent Cochranereview provides no evidence for exercise effects on vasomotor symptoms15 [51-53]. However, another review demonstrates that exercise improves sleep quality, an aspect also included in the somatovegetative complex of the MRS [54]. Thus, it might be more accurate to address and evaluate single menopausal symptoms and complaints rather than summarize them in dimensions or complexes.
There is considerable evidence that back pain in particular tends to increase during the menopause transition and earlypostmenopause [55]. Dedicated resistance and stabilization-type exercise is a recognized therapy for chronic or subacute low back pain [56,57]. Although ACTLIFE-ER suffers from a floor effect16, we confirmed these data by observing significant positive effects on LBP pain frequency and severity.
Finally, we determined significant effects on maximum hip-/leg extensor strength and power (ie maximum jumping height) that averaged 15-20%. However, these changes were lower compared with DRT trials (30-85%) with women in the range of the early menopause [43,44,58-61]. We attribute this result in part, to rather high baseline values in our cohort.
Some particularities and limitations of ACTLIFE-ER should be addressed to allow the reader to adequately comprehend our interpretation of the results. (1) From a biometric point of view, one may argue that we addressed too many study endpoints. However, the primary study aim of ACTLIFE-ER is to determine the effect of a tailored exercise protocol on changes related to early menopause with specific regard for BMD. We do not include dedicated cardiometabolic and cardiovascular risk factors that are closely related to estrogen declines in the present analysis, this is the subject of a more specific analysis [62]. (2) We introduced an active control group not to blind participants17 but to give women in the CG an opportunity to exercise. It should be noted that we opted for frank communication concerning the pros and contras of both groups, which can be considered as the reason for 21 women refusing to be randomly assigned to the groups. Though the CG performed exercises with low exercise intensity and training frequency, we have to accept that the exercise protocol of the CG might have affected some of the study endpoints addressed. Thus, there is some evidence that effects generated by our study protocol might be more discreet compared to an approach with an “inactive” CG. (3) BMD-LS at 7month FU was provided by a whole-body DXA scan and not by a dedicated DXA or a quantitative computed tomography scan of the LS area. The FAU ethics committee specified this limitation in order to reduce x-ray exposure. (4) We did not use more reliable pain diaries for the assessments of low back pain but asked for frequency and severity of pain episodes during the last 4 weeks. (5)Both energy and particularly dietary protein intake was in the upper range of German women 51-64 years old (Energy: 1850 kcal/d, 50% CI: 1500-2100 kcal/d, protein: 68 g/d, 50% CI: ≈55-80 g/d) [63]. (6) Drawing lots might not be the most sophisticated randomization strategy. Nevertheless, our experience shows that this strategy, along with a detailed information about the characteristics of EG and CG, increase adherence, particularly after self-allocation to the non-favored study arm.
In conclusion, we demonstrated the general effectiveness of a multipurpose exercise protocol on various aspects negatively impacted by the menopausal transition. Future assessments within the ACTLIFE-ER project have to determine the exercise effect on Bone Mineral Density that might be the most challenging physiologic outcome within our study endpoints.
Acknowledgements
This study is one of the intellectual outputs of the project “ACTLIFE-Physical activity: the tool to improve the quality of life in osteoporosis people” conducted by a consortium of researchers from Italy, Finland, Germany, UK, Ireland and Bulgaria.
None to report
The study was funded from the European Union’s Erasmus Plus Sport program under grant agreement No. 2017-2128/001-001.
1According to German recommendations [20], women with a higher risk (T-Score >4 SD) are entitled to pharmaceutic therapy.
2Eg Glucocorticoids >7.5 mg/d>3 months (n=12), Thyroxin >7.5 mg/d>3 months (n=11).
3Eg Morbus Cushing (n=1), hyperthyroidism (n=13), severe arthritis of knee or hips (n=9)
4Eg aerobic dance, volleyball, tennis, Pilates, calisthenics, however, jogging was not excluded.
52-3 cycles of 4-5 weeks with each 4-5th week as a “recovery” week with low intensity.
62-3 cycles of 4-5 weeks with each 4-5th week as a “recovery” week with low intensity.
7“Set endpoint when trainees complete the final repetition possible whereby if the next repetition was attempted they would definitely achieve MF” [22].
85-20 reps at nRM-1 rep corresponding to 65-82.5% 1RM [23].
9Participants were asked to do not exceed a pleasant feeling of tension.
10In parallel to DXA, however, data reported here refer to the DXA-assessment.
11In parallel to DXA, however, data reported here refer to the DXA-assessment.
12Corresponding results were observed for maximum hip/leg flexors strength p<.001, SMD: 0.98)
13Since none of the studies apart from Karakiriou et al. address women in their early-postmenopausal years, i.e. Women with increased bone loss, the pronounced 6-7 month decreases in the CGs were surprising [40]. Further, in some cases results for BMD-LS changes (e.g. EG: 15.8% vs CG: -8.5%) might be hard to find realistic [42].
143x 45-60 min/week, progressive, block-periodized mixed exercise, but with a high aerobic exercise component
15However, only data for hot flushes/night sweats were subsumed under “vasomotor symptoms”.
16Only 37 participants reported to have suffered from low back pain within the last month
17Considering personal relations between the participants, “blinding” is not only unrealistic, but also counterproductive since participants of the CG might withdraw, due to the loss of confidentiality.
Citation: Kemmler W, Hettchen M, Kohl M, Murphy MH, Shojaa M, Ghasemikaram M, et al. (2020) Effects of High Intensity Exercise during Early Postmenopause-the Randomized Controlled ACTLIFE-Study. J Osteopor Phys Act 8:228. doi: 10.35248/2329-9509.20.8.228.
Received: 01-Sep-2020 Accepted: 23-Sep-2020 Published: 30-Sep-2020 , DOI: 10.35248/2329-9509.20.8.228
Copyright: © 2020 Kemmler W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : The study was funded from the European Unionâ??s Erasmus Plus Sport program under grant agreement No. 2017-2128/001-001.