Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research Paper - (2019)Volume 10, Issue 3
Clonal propagation is a process of asexually reproducing plants by multiplication of one copy to produce several copies that are genetically identical. One of the best methods of clonal propagation is using the cuttings. There are many plants that can be propagated through cuttings and one of them is guava. Guava is popular in Pakistan. To ensure that a guava tree will produce fruits that belong to its original cultivar, this study was conducted. Softwood cuttings of guava were treated with IBA (1600 ppm and 2000 ppm), NAA (7000 ppm and 10,000 ppm) and IBA+NAA (1600+7000 ppm and 2000+10,000 ppm). There were five media used (Soil, Peatmoss+Soil, Sand+Soil, Baggasse+Soil and Soil+Peatmoss+Sand+Bagasse. The experiment was laid out using Completely Randomized Block Design (CRBD) with two-factor factorial arrangement. A total of 105 cuttings were used, each with 12 cm length and with 2-4 nodes. Media samples were collected and physio-chemically analyzed at the Soil and Water Testing Laboratory in Layyah, Pakistan to assess the properties and fertility status. Guava cuttings with 1600 ppm IBA showed the best results in terms of the number of roots, root length and days to sprout, followed by 7000 ppm NAA in Peat moss+soil and soil+peat mass+sand+bagasse. For the combination of growth regulators, the best results were observed in IBA 1600 ppm + NAA 7000 ppm. Poorest result in all parameters (days to sprout, number of roots, and root length) was recorded in control. The 1600 ppm IBA with 10 second dipping time for softwood cuttings of guava yielded best results in peatmoss+soil media for all the parameters tested. Hence, this study concluded that treatment with auxin (such as IBA) can yield best results and can help to solve the problem in propagating guava using cuttings.
Guava; IBA; Cutting; Rooting; Peatmoss
Guava (Psidium guajava L.) belongs to family Myrtaceae. It is an evergreen plant which is commercially grown in the tropics and sub-tropics [1,2]. It originated from Central America and the southern regions of Mexico. Family Myrtaceae include approximately 130 genera and 3,000 species. The genus Psidium consists of more than 152 species which include evergreen trees and shrubs which are widely propagated in different tropics and sub-tropics [3]. Guava plants are resistant to salt and drought. Guava fruits contain ascorbic acid, iron, phosphorus and calcium and they have 2-3 times more vitamin C than oranges [4]. A 100 g of guava fruit contains 260mg of vitamin C [5]. Pakistan is the 2nd largest guava producing country. It is grown almost in all provinces; predominately Punjab while other major growing areas include Lahore, Gujranwala, Sahiwal, Sheikhupora, kasur, Faisalabad and Jhang. Commercial guavas are propagated sexually through seeds in which plants cannot preserve the characteristics of the variety due to segregation. This has given rise to the selection of several promising landraces. ‘Sharakpur’ is the result of such seedling selection of guava in Pakistan. Mostly in Pakistan, it is propagated through seed but segregation occurs due to cross pollination [6]. The most common vegetative propagation methods used in guava are layering, budding, grafting and cuttings [7]. Softwood cuttings of Gola variety of guava can be rooted by quick dip method with dipping the basal portion of cuttings in a mixture of Indole 3-butyric acid under low tunnel shady conditions [8]. Among the vegetative methods of propagation, rooting is undoubtedly expanded and the most evolved method [9]. Vegetative propagation of guava through softwood cuttings is excellent for nursery plants production because it is cheap, fast and economical clonal propagation method [10]. Auxin is relatively helpful among cuttings to overcome difficulties in root induction. Indole butyric acid (IBA) is a plant growth hormone among the auxin family and is used in many horticultural plants for root induction [10,11]. Naphthalene acetic acid (NAA), Indoleacetic acid (IAA) and 2, 4-dichloro-phenoxyacetic acid is also included in the auxin family which is commonly used for rooting. It has been established that auxins are essential for root development [12]. This study aimed to develop an asexual propagation method for guava and to determine the best rooting media. This study also aimed to identify the optimum concentration and combination of auxins for guava rooting under low-plastic tunnel.
The research was conducted in the Nursery area of the College of Agriculture, BZU, Bahadur sub-campus in Layyah, Pakistan from March to July in 2017. The guava var. Chinese Gola was accessed and selected as plant material for clonal propagation. Softwood cuttings of guava were taken from fruiting trees of a 10-year-old Chinese Gola variety tree. Softwood cuttings measuring 12 cm long with two to four nodes were being prepared from the apical portion of the shoots. The leaves were removed completely. There were five media used including soil, peatmoss + soil, sand + soil, bagasse + soil, soil + peatmoss + sand + bagasse (Table 1). Six treatments of auxin were used in the study: IBA (2000 ppm and 1600 ppm), NAA (7000 ppm and 10,000 ppm) and combinations of IBA and NAA (IBA 1600 ppm + NAA 7000 ppm) and (IBA 2000 ppm+ NAA 10,000 ppm). The lower portions of the cuttings were dipped up to 2-3 cm in auxin treatments for 10 seconds. The treated cuttings were inserted into the rooting media under low-plastic tunnel for 2-3 cm in depth. A total of 105 cuttings were planted. After planting the cuttings, the irrigation was applied immediately. Media samples were collected and were analysed for physio-chemical properties and fertility status (N, P, K, O, M, pH, Gypsum requirement, texture and Electrical Conductivity). Finally, the research parameters such as the day to sprout, number of leaves per cutting, number of roots per cutting and root length were recorded. The research design used was Completely Randomized Block design (RCBD) with 2-factor factorial arrangement. The data were statistically analyzed using Statistix 8.1(Software) and the means were analyzed using LSD test (Table 2 and Figure 1).
| Combinations | Treatments | Ratio by volume |
|---|---|---|
| Soil | M1 | 1 |
| Soil+ peat moss | M2 | 1:1 |
| Soil+ sand | M3 | 1:1 |
| Soil+ baggasse | M4 | 1:1 |
| Soil+peatmass+sand+bagass | M5 | 1:1:1:1 |
Table 1: Different combinations of growing media used.
| Growth regulators | Treatment | Concentration (ppm) |
|---|---|---|
| Control | To | Control (Distil water) |
| IBA | T1 | 1600 |
| IBA | T2 | 2000 |
| NAA | T3 | 7000 |
| NAA | T4 | 10,000 |
| IBA+NAA | T5 | 1600+7000 |
| IBA+NAA | T6 | 2000+10000 |
Table 2: Combinations of IBA and NAA treatments used in the study.
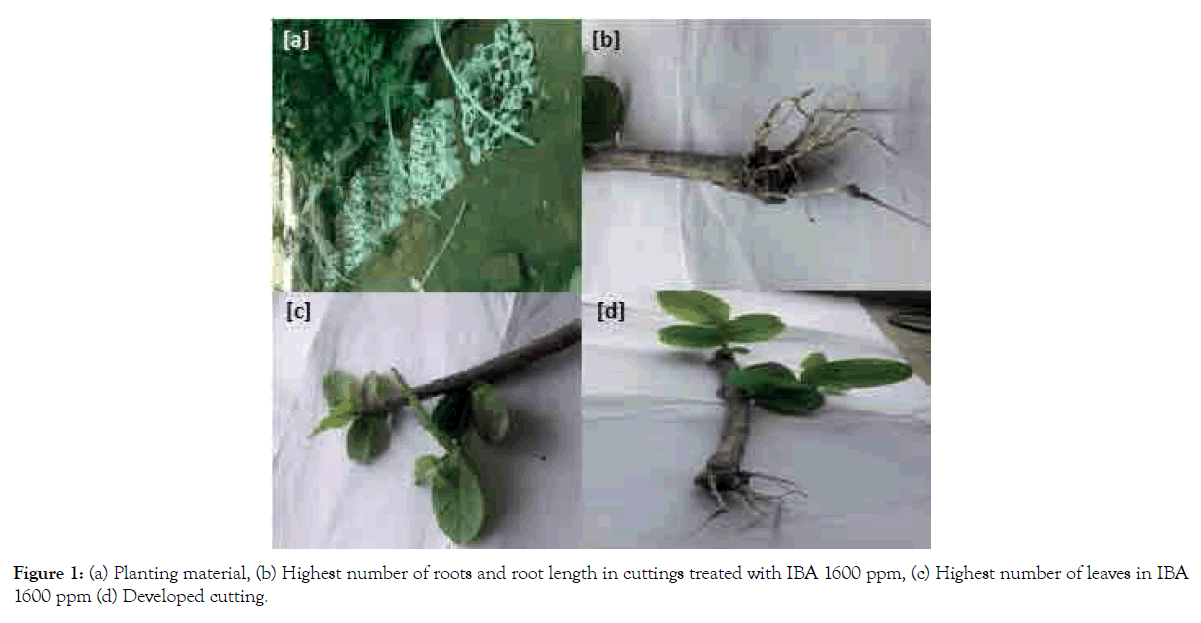
Figure 1: (a) Planting material, (b) Highest number of roots and root length in cuttings treated with IBA 1600 ppm, (c) Highest number of leaves in IBA 1600 ppm (d) Developed cutting.
Effect of IBA and NAA in soil media
The results for the days to sprout and number of roots are statistically non-significant. On the other hand, significant results were observed in number of leaves and root length. As shown in Table 3, the highest number of leaves (5.0) were observed in T1 (IBA 1600 ppm) followed by T3 (NAA 7000 ppm) with 4.3 number of leaves. Meanwhile, the longest root length (3.5 cm) was recorded in T1 followed by T5 (IBA+NAA 1600+7000 ppm) (3.4 cm). The lowest number of leaves and shortest route length were recorded in control (distilled water). However, these results were not as good as the other media due to the less quantity of organic matter or less porosity and soil aeration.
| Treatment (ppm) | Days to sprout | No. of leaves | No. of roots | Root length (cm) |
|---|---|---|---|---|
| T0 T1 T2 T3 T4 T5 T6 |
18.3 22.0 21.0 25.0 19.0 21.6 20.0 |
2.3c 5.0a 3.6abc 4.3a 2.6bc 4.0ab 2.3c |
4.3 3.6 4.0 5.0 5.6 5.0 3.3 |
1.1b 3.5a 1.7b 3.0a 2.0b 3.4a 1.4b |
| F-value P-value |
1.35 0.300 n.s |
8.71 0.0005* |
2.73 0.05 n.s |
18.4 0.000** |
T0= (distil water); T1= IBA (1600 ppm); T3=NAA (7000 ppm); T5= IBA+NAA (1600+7000 ppm)
T2, IBA (2000 ppm); T4= NAA (10,000 ppm); T6= IBA+NAA (2000+10000 ppm).
(a) Planting material; (b) Highest number of roots and root length in cuttings treated with IBA 1600 ppm; (c) Highest number of leaves in IBA 1600 ppm; (d) Developed cutting.
Table 3: Effect of IBA and NAA on days to sprout number of leaves, number of roots and root length in guava cuttings in soil media.
Effect of IBA and NAA in peatmoss+soil media
The collected data were statistically analyzed at 1% probability level. As shown in Table 4, significant results were observed in T 1 (IBA 1600 ppm) which gave the highest average number of leaves (13.6), highest number of roots (15.0) and highest root length (13.1 cm) per plant followed by T3 (NAA 7000 ppm). Rooting hormones application enhanced the capability of root formation as described by many authors [8,13-18]. There were non-significant differences between T2 (IBA 2000 ppm) and T5 (IBA+NAA 1600+7000 ppm) in terms of the number of leaves. Growth regulators may indirectly affect the number of leaves because of nutrients uptake. Auxins establish stronger root systems which are efficient in nutrient uptake. These results are similar to Akram coworkers which showed that IBA produced the highest number of leaves [18]. Meanwhile, the results of this study were also similar to El-Sayed coworkers who established that vegetative growth was best due to peatmoss application as growing media. Among the combinations, T5 showed best results in all the parameters. In terms of early sprouting, the least number of days (17.6) to sprout was also observed in T1 (IBA 1600 ppm). Perhaps, it was due to the presence of early differentiated cells or due to the effect of growth regulator (IBA). These results are supported by Kareem coworkers where higher concentration of IBA (4000 ppm) took the least days to sprout [8]. However, in this study, T6 (IBA+NAA 2000+10,000 ppm) took more days to sprout (24.3). Delay in sprouting in T6 may be due to the mass of dry matter. Phytotoxity can also be a reason of growth inhibition [19]. On the other hand, the control (distilled water T0) produced the poorest results in terms of all parameters tested (Table 4).
| Treatment (ppm) | Days to sprout | No. of leaves | No. of roots | Root length (cm) |
|---|---|---|---|---|
| T0 T1 T2 T3 T4 T5 T6 |
24.0ab 17.6a 20.0bcd 19.6cd 22.0abc 18.0cd 24.3a |
5.3d 13.6a 11.0b 13.0ab 8.3c 11.3b 8.0c |
3.0d 15.0a 8.6b 14.6a 6.0c 10.0b 7.6bc |
1.7e 13.1a 7.3c 11.5ab 4.8d 9.6b 6.0cd |
| F-value P-value |
6.96 0.001* |
29.7 0.000** |
49.6 0.0000** |
70.1 0.0000** |
(a) Planting material; (b) Highest number of roots and root length in cuttings treated with IBA 1600 ppm; (c) Highest number of leaves in IBA 1600 ppm; (d) Developed cutting.
Table 4: Effect of IBA and NAA on days to sprout, number of leaves, number of roots and root length in Peatmoss+Soil media.
Effect of IBA and NAA in sand+soil media
As shown in Table 5, significant results were recorded in the cuttings treated with the growth regulators. The least number of days to sprouting (19.6) were recorded in T1 (IBA 1600 ppm). On the other hand, the control (distilled water T0) took the highest number of days in sprouting which may be due to absence of the application of any treatment. The highest number of leaves (8.0) and highest number of roots (9.0) were observed in T3 (NAA 7000 ppm) followed by T1 (IBA 1600 ppm) with an average number of leaves of 7.6 and an average number of roots of 7.0. Results showed that IBA with sand medium does not perform better than peatmoss medium. These results are supported by Akram [18]. The results in terms of the numbers of roots are similar to the study of Rahman coworkers in which the IBA was established as a root promoting hormone; it induces root formation by stimulating the cambium activity [20]. Moreover, the root growth was good due to sand application as growing media similar to the reports of [21]. The good root growth was due to porosity and aeration of the media. The longest root length (9.3 cm) was recorded in T1 (IBA 1600 ppm) which was similar to the study by [22]. Treatment 3 also showed significant results in terms of root length (8.2 cm) following T1. Among the combinations of treatments, T5 (IBA+NAA 1600+7000 ppm) showed the best results. Ramtin coworkers reported that the medium with lower capacity of water retention will produce longer roots [23]. Meanwhile, the least number of leaves, number of roots and root length were observed in the control (distilled water T0) as shown in Table 5. The control did not yield good results which established the correlation between treatment and media.
| Treatment (ppm) | Days to sprout | No. of leaves | No. of roots | Root length(cm) |
|---|---|---|---|---|
| T0 T1 T2 T3 T4 T5 T6 |
25.6ab 19.6c 26.0ab 21.6bc 28.3a 23.0bc 25.0ab |
3.0c 7.6a 5.6abc 8.0a 5.3abc 6.0ab 4.6bc |
1.6e 9.0a 4.0cd 7.0ab 5.0bcd 6.0bc 3.0de |
2.0e 9.3a 7.7ab 8.2ab 4.0de 6.3bc 5.4cd |
| F-value P-value |
6.70 0.0017* |
6.64 0.0017* |
24.3 0.0000** |
28.1 0.0000** |
(a) Planting material; (b) Highest number of roots and root length in cuttings treated with IBA 1600 ppm; (c) Highest number of leaves in IBA 1600 ppm; (d) Developed cutting.
Table 5: Effect of IBA and NAA on days to sprout number of leaves, number of roots and root length in sand+soil media.
Effect of IBA and NAA in baggasse+soil media
As shown in Table 6, the results were statistically significant. The results for days to sprout showed that T1 (IBA 1600 ppm) took the least number of days (21.3) to sprout as compare with other treatments. There were non-significant differences between T1 and T5 (21.6) which had the second to the least number of days to sprout. The cuttings planted in T6 (IBA+NAA 2000+10,000 ppm) had the highest number of days (30.6 d) to sprout. This may be due to the higher flow of metabolites towards the differentiating growing bud that prolonged the sprouting of the cuttings [8]. In terms of the number of leaves, number of roots and root length, T1 (IBA 1600 ppm) had the highest significant results with 9.0, 8.0 and 9.3, respectively. Tyagi and Patel and Lal coworkers reported that IBA can yield significant results compared with NAA [22,24]. IBA application can also yield positive results in terms of number of roots [13]. Treatment 2 (IBA 2000 ppm) and T3 (NAA 7000 ppm) showed non -significant differences in terms of the number of leaves (6.3 and 6.0). In terms of root length, the best results were observed in treatment 5 (IBA+NAA 1600+10,000 ppm) with 8.7 cm. These results were confirmed by Sen who reported that the least number of roots can be observed in control but not in the treatments [25]. Furthermore, the lowest number of leaves in control was due to no application of IBA and NAA. The medium can also be the possible reason because bagasse+soil media yielded the poorest results (Table 6).
| Treatment (ppm) | Days to sprout | No. of leaves | No. of roots | Root length(cm) |
|---|---|---|---|---|
| T0 T1 T2 T3 T4 T5 T6 |
27.3ab 21.3c 27.0ab 23.3bc 25.3bc 21.6c 30.6a |
1.3e 9.0a 6.3bc 6.0bcd 4.0cd 7.0ab 3.6de |
1.3d 8.0a 6.0abc 7.0ab 4.0c 6.3abc 5.0bc |
2.7d 9.3a 3.3cd 6.0bc 4.4cd 8.7ab 5.0cd |
| F-value P-value |
8.81 0.0004* |
19.1 0.0000** |
13.3 0.0000** |
14.7 0.0000** |
(a) Planting material; (b) Highest number of roots and root length in cuttings treated with IBA 1600 ppm; (c) Highest number of leaves in IBA 1600 ppm; (d) Developed cutting.
Table 6: Effect of IBA and NAA on days to sprout number of leaves, number of roots and root length in Baggasse+Soil media.
Effect of IBA and NAA in mixture of all media
As shown in Table 7, the results recorded in this media were statistically significant. In terms of the number of days to sprout, the least number of days was observed in T1 (IBA 1600 ppm) with 18.0, followed by T3 (NAA 7000 ppm) with 19.3 and T5 (IBA+NAA 1600+7000 ppm) with 21.0. Wahab coworkers reported that bud sprouting is attributed to carbohydrates stored in the cuttings [13]. Moreover, the application of auxins may have indirect effects to the cuttings such as increasing the number of roots, root length and may trigger or initiate the production of root promoting chemical like radiocarbon in the roots which may play vital role in sprouting. On the other hand, T6 (IBA+NAA 2000+10,000 ppm) had the highest number of days to sprout days followed by the control (distilled water T0). In terms of the number of leaves and roots, significant results were reported in T1 (IBA 1600 ppm) which yielded 13.0 and 15.0, respectively. This was followed by T3 (NAA 7000 ppm). The optimum concentration of IBA may cause the mobilization and utilization of carbohydrates, nitrogen fraction, and absorption of water and mineral nutrients [26,27]. Among the combinations, T5 (IBA+NAA 1600+7000 ppm) showed the best results (11 and 12.3). Meanwhile, the longest root length (13.0 cm) was recorded in T3 (NAA 7000 ppm) followed by T1 (IBA 1600 ppm) with an average root length of 12.1 cm. These results are similar to the study by McGuire coworkers [28]. Auxin induces the rooting of the cuttings as shown in several studies [12,13,29]. On the other hand, the poo Psidium rest results were observed in the control. Overall, the different concentrations and combinations of growth regulators showed better results compared with the control. IBA 1600 ppm was the best concentration of growth regulators followed by NAA 7000 ppm in terms of all the parameters tested [30-33].
| Treatment (ppm) | Days to sprout | No. of leaves | No. of roots | Root length(cm) |
|---|---|---|---|---|
| T0 T1 T2 T3 T4 T5 T6 |
30.3ab 18.0d 23.3c 19.3cd 28.6b 21.0cd 31.0a |
5.3d 13.0a 10.0bc 12.3ab 7.0d 11.0ab 7.3cd |
3.3e 15.0a 9.6c 13.3ab 6.3d 12.3b 7.0d |
3.4d 12.1ab 8.6c 13.0a 5.8d 10.0bc 5.2d |
| F-value P-value |
37.2 0.0000** |
19.9 0.0000** |
49.0 0.0000** |
33.5 0.0000** |
(a) Planting material; (b) Highest number of roots and root length in cuttings treated with IBA 1600 ppm; (c) Highest number of leaves in IBA 1600 ppm; (d) Developed cutting.
Table 7: Effect of IBA and NAA on days to sprout number of leaves, number of roots and root length in guava cuttings in mixture of all media.
Propagation of guava through cuttings can yield the best results in terms of the number of days to sprout, number of roots, and root length with the application of 1600 ppm of IBA followed by 7000 ppm of NAA in peatmoss+soil and soil+peatmoss+sand+bagasse. NAA application had positive effect on guava rooting. Results of rooting in the guava cuttings are promising and that it revealed the potential of this clonal propagation technique in guava for elite genotypes. Furthermore, this technique is simpler and cheaper and can be recommended even to nursery growers.
Citation: Shahzad U, Kareem A, Altaf K, Zaman S, Ditta A, Yousafi Q, et al. (2019) Effects of Auxin and Media Additives on the Clonal Propagation of Guava Cuttings (Psidium guajava L.) Var. Chinese Gola. J Agri Sci Food Res. 10: 265. doi: 10.35259/2593-9173.19.10.265
Received: 28-Jun-2019 Accepted: 11-Sep-2019 Published: 17-Sep-2019 , DOI: 10.35248/2593-9173.19.10.265
Copyright: © 2019 Shahzad U, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.