Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2020)Volume 11, Issue 4
Objective: There is controversy surrounding the next best step in treating plateau iris syndrome (PIS) patients with
persistent angle dysfunction despite a patent laser peripheral iridotomy (LPI). The aim of this study was to examine
the effectiveness of argon laser peripheral iridoplasty (ALPI) in PIS patients with a patent LPI.
Methods: Retrospective review of medical records in consecutive patients that underwent ALPI to treat persistently
narrow angles due to underlying PIS after a patent LPI. Patients in whom angle configuration could not be
ascertained by review of medical records, those with less than 12 months follow-up at our institution, or those
younger than 18 years of age were excluded. Kaplan-Meier survival analysis was used to determine treatment survival
time. Paired t-tests were used to compare intraocular pressure (IOP), number of glaucoma medications, and bestcorrected
visual acuity (BCVA) 12 months post-ALPI and at the last follow-up visit.
Results: Fifty-one eyes of 51 patients were included in the analysis. The median survival time for a successful ALPI
procedure was 1023 days (34.1 months). The majority of failure events were attributed to cataract extraction to open a
persistently narrow angle, performed at approximately 934 (± 694) days (31.1 months) after ALPI. No changes in IOP,
number of glaucoma medications, or BCVA from baseline were observed after 12 months or at last follow-up.
Conclusion: ALPI is a potentially effective treatment for PIS following LPI. While angle dysfunction may re-emerge
over time, ALPI can potentially delay the need for lensectomy in patients without visually significant cataracts.
Argon Laser Peripheral Iridoplasty (ALPI); Laser Peripheral Iridotomy (LPI); Glaucoma; Plateau Iris Syndrome (PIS); Angle Dysfunction; Lensectomy
There is continued controversy surrounding the next best step in the treatment of a patient with persistently narrow angles after a patent laser peripheral iridotomy (LPI). Treatment options include argon laser peripheral iridoplasty (ALPI), cataract extraction, or observation, depending on the individual clinical scenario. Optimizing the treatment algorithm would be highly impactful as it is estimated that by 2021, 5.3 million people will suffer from blindness due to primary angle-closure glaucoma [1].
Relative pupillary block is often regarded as the most common mechanism of primary angle closure, in which LPI would both open the angle and lower intraocular pressure (IOP) [2,3]. However, it has been reported that approximately one-third of patients present with persistent angle dysfunction despite a patent LPI [2,3]. This is often observed in cases of plateau iris syndrome (PIS), a mechanism of angle-closure that persists despite a patent LPI.
In 1977, Wand and colleagues differentiated plateau iris configuration (anteriorly positioned ciliary body resulting in angle closure prior to intervention) from plateau iris syndrome (PIS, plateau iris configuration that persists despite LPI) [4]. ALPI was first described by Ritch in 1982, and it has since been considered a potentially effective treatment for persistently narrow angles after a failed LPI [5]. ALPI delivers continuous wave laser energy to the peripheral iris causing the iris tissue to contract, thereby pulling it away from the TM and opening the angle. However, to date only a limited number of studies have investigated the efficacy of ALPI, with sample sizes of 22 to 48 eyes and variable follow up between 76 and 92 months [6-8]. Furthermore, factors affecting the survival of ALPI efficacy have not been previously reported. In this study, we performed a survival analysis on the effect of ALPI in order to elucidate factors associated with failure.
Approval for this retrospective review was obtained from the Partners Healthcare Institutional Review Board. All research activities adhered to the tenets of the Declaration of Helsinki and was compliant with the Health Information Portability and Accountability Act. Consecutive patients who underwent ALPI were identified using financial claims data (Current Procedural Terminology code 66762) between 2013 and 2018. Patients were included in the analysis if they had documented PIS (defined below) and a patent LPI prior to ALPI, and at least 12 months of follow-up at our institution following ALPI. Patients were excluded from the analysis if they were not 18 years of age, or if angle configuration could not be ascertained by review of medical records. If both eyes were treated, only the right eye was selected for the analysis.
Study definitions
Plateau iris syndrome (PIS): inability to visualize posterior trabecular meshwork (PTM) in more than 2 quadrants on gonioscopy post-LPI, ultrasound biomicroscopy findings indicating PIS, post-dilation IOP spike (≥ 10%), or as indicated by clinical diagnostic codes.
Baseline IOP: baseline IOP was calculated as the average of the values at the two most recent pre-ALPI visits.
Failure criteria: inability to visualize PTM in more than 2 quadrants on gonioscopy (gonioscopic failure), an acute-angle closure attack, lens surgery for the purpose of improving angle function, or an increase in IOP ≥ 20% from pretreatment baseline at 2 consecutive visits.
Censor criteria: Reaching the end of the follow-up period, lens surgery for visually significant cataracts, or the addition of glaucoma medications from pretreatment baseline without first meeting the failure criteria for uncontrolled IOP. If failure and censoring criteria were reached on the same day, failure was declared.
Main outcome measures
The main outcome measure was Kaplan-Meier survival using the above defined failure criteria. Secondary outcomes included IOP, number of glaucoma medications, and best-corrected visual acuity (BCVA) assessed at 12 (± 4) months post-ALPI and at the last follow-up visit.
ALPI procedures
ALPI was performed by multiple glaucoma specialists with similar technique as described elsewhere [5].
Data analysis
Demographic and baseline clinical data were summarized by mean (± standard deviation) or frequency (percentage) for categorical variables. Snellen BCVA was converted to the logarithm of the minimum angle of resolution (logMAR) for analysis. Paired t-tests were used to compare changes in IOP, number of glaucoma medications, and logMAR visual acuity from baseline to 12 (± 4) months and last follow-up. Any patient that did not have a follow-up visit within 12 (± 4) months was excluded from the 12-month analysis. IOP and medication data for patients that underwent an IOP-lowering procedure prior to the 12-month visit or last follow-up were excluded from the IOP and medication analyses. Visual acuity data for patients that underwent phacoemulsification prior to the 12-month visit or last follow-up were excluded from the BCVA analyses.
A Kaplan-Meier curve was created based on the above defined failure criteria. A Cox Proportional-Hazards model was utilized to determine the effect of baseline characteristics on the likelihood of survival. All assumptions required for the Cox Proportional-Hazards model were met. Fifteen patients were selected at random to assess the frequency with which patients underwent gonioscopy throughout their follow-up. All statistical tests were conducted at a 5% level of significance. Analysis was performed using R statistical programming software (version 3.6.2).
A total of 64 patients were screened, and 51 eyes from 51 patients were included in the analysis. Patient demographics are given in Table 1. The median age was 59 [IQR 15] years and the mean age was 57.3 (± 10) years. The median follow-up period was 1385 [IQR 1023] days (46.2 months) and the mean followup was 1328 (± 654) days (44.3 months). Average laser settings were as follows: spot size of 389 (± 160) microns, power of 256 (± 105) milliwatts, and a duration of 473 (± 160) milliseconds. An average of 30 (± 10) evenly spaced spots were placed over 360 degrees.
| Median Age [IQR] (years) | 59 (15) |
|---|---|
| Sex % (n) | |
| Female | 67 (34) |
| Male | 33 (17) |
| Race % (n) | |
| White | 72 (37) |
| Black or African American | 6 (3) |
| Hispanic | 14 (7) |
| Asian | 4 (2) |
| Pacific Islander | 2 (1) |
| Unknown | 2 (1) |
Median age [interquartile range] and distribution of sex and race amongst study participants.
Table 1: Patient demographics.
Baseline characteristics
Thirty-five percent of patients had a glaucoma diagnosis at the time of ALPI. The distribution of glaucoma diagnoses, as well as baseline IOP, number of glaucoma medications, and logMAR BCVA are given in Table 2. The mean baseline IOP was 16.5 (± 4.8) mmHg. The mean number of glaucoma medications was 0.82 (± 1.3). Twenty (39%) eyes used at least one glaucoma medication to control IOP. Seven (35%) of these 20 patients were using pilocarpine. The mean logMAR visual acuity was 0.08 (± 0.2). Ten (19.6%) patients had peripheral anterior synechiae (PAS) present preoperatively.
| PIS patients with glaucoma % (n) | |
|---|---|
| Glaucoma diagnoses % (n) | 35 (18) |
| CACG | 29 (15) |
| MMG | 6 (3) |
| Baseline IOP (± sd) (mmHg) | 16.5 (± 4.8) |
| Baseline medications (± sd) | 0.82 (± 1.3) |
| Baseline logMAR BCVA | 0.08 (± 0.2) |
Note: Baseline characteristics amongst study participants. PIS: Plateau Iris Syndrome; CACG: Chronic Angle-Closure Glaucoma; MMG: Mixed-Mechanism Glaucoma; IOP: Intraocular Pressure; BCVA: Best Corrected Visual Acuity was converted to logMAR: Logarithm of the Minimum Angle of Resolution.
Table 2: Baseline characteristics.
Outcomes
Kaplan-Meier survival analysis: The Kaplan-Meier survival function is shown in Figure 1. The median survival time for a successful ALPI procedure was 1023 days, or approximately 34.1 months. The cumulative probability of success was 70% (95% CI 0.58-0.84) and 56% (95% CI 0.43-0.72) at 12 months and 24 months, respectively. There were 29 failure events in total. Cataract surgery to open the angle with or without additional IOP-lowering measures accounted for 13 failure events while gonioscopic failure accounted for 8. Elevated IOP ≥ 20% from baseline accounted for 7 failure events and an acute angle closure attack for 1. Of those who failed for reasons other than surgical intervention, 8 would go on to have cataract surgery with or without adjunctive IOP-lowering measures to treat progressive angle dysfunction.
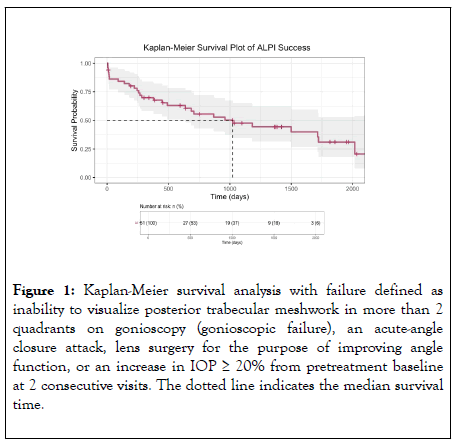
Figure 1: Kaplan-Meier survival analysis with failure defined as inability to visualize posterior trabecular meshwork in more than 2 quadrants on gonioscopy (gonioscopic failure), an acute-angle closure attack, lens surgery for the purpose of improving angle function, or an increase in IOP = 20% from pretreatment baseline at 2 consecutive visits. The dotted line indicates the median survival time.
As previously mentioned, the frequency at which patients had gonioscopy was assessed in a subset of 15 randomly selected patients. Gonioscopy was performed on all 15 patients at their first follow-up visit after ALPI. The median number of times gonioscopy was performed throughout these patients’ follow-up was 4 [IQR 2.5], over a median of 1441 [IQR 888] days, or approximately 48 months.
A Cox Proportional- Hazards analysis was performed to investigate predictors of failure (Table 3). Neither sex, non-white race, nor age were significant predictors of failure (p=0.7, 0.7, 0.2, respectively). Pretreatment baseline IOP, medications, BCVA, and diagnosed glaucoma were also not significant predictors of failure (p=0.2, 1, 0.3, 0.1, respectively).
| Covariate | HR | 95% CI | p value |
|---|---|---|---|
| Sex | 1.18 | 0.54-2.56 | 0.7 |
| Non-white race | 1.18 | 0.53-2.58 | 0.7 |
| Age | 1.03 | 0.99-1.06 | 0.2 |
| Baseline IOP | 0.95 | 0.88-1.03 | 0.2 |
| Baseline glaucoma medications | 1 | 0.74-1.36 | 1 |
| BCVA | 2.11 | 0.52-8.52 | 0.3 |
| Diagnosed glaucoma | 0.52 | 0.23-1.14 | 0.1 |
HR: Hazard ratios for baseline covariates from the Cox-Proportional Hazards model reported with 95% CI: Confidence Intervals; IOP: Intraocular Pressure; BCVA: Best Corrected Visual Acuity
Table 3: Hazard ratios for ALPI failure risk factors.
Intraocular pressure, number of glaucoma medications, and visual acuity
Forty-three of 51 patients had a follow-up visit within 12 (± 4) months from their initial ALPI procedure with no additional IOP-lowering procedure and were therefore included in the analysis of IOP and medications. There were no differences between baseline and 12-month IOP (p=0.99) or medications (p=0.52) (Table 4). Forty-two patients had a follow-up visit within 12 (± 4) months without prior phacoemulsification, and they were therefore included in the comparison of BCVA means. There was no significant difference in logMAR BCVA at 12 months compared to baseline (p=0.05) (Table 4).
| IOP (± sd) (mmHg) | Medications (± sd) | logMAR BCVA (± sd) | |
|---|---|---|---|
| Baseline | 17.1 (4.6) | 0.67 (1.0) | 0.08 (0.21) |
| 12 months | 17.2 (5.3) | 0.74 (1.) | 0.18 (0.41) |
| n | 43 | 43 | 42 |
| p value | 0.99 | 0.52 | 0.05 |
| Baseline | 16.7 (5.0) | 0.56 (0.97) | 0.03 (0.09) |
| Last follow-up | 15.8 (3.2) | 0.44 (0.84) | 0.17 (0.44) |
| n | 45 | 45 | 26 |
| p value | 0.22 | 0.32 | 0.07 |
Intraocular pressure (IOP), glaucoma medications, and logarithm of the minimum angle of resolution (logMAR) best-corrected visual acuity (BCVA) at baseline, 12 (± 4) months, and last-follow-up. Mean time to last follow-up was 45 ± 22 months. Patients that had an additional IOP-lowering procedure prior to the 12-month mark or last follow-up were excluded from the IOP and medication comparison of means. Patients that had phacoemulsification prior to the 12-month mark or last follow-up were excluded from the BCVA comparison of means.
Table 4: IOP, medications, and visual acuity at baseline, 12 months, and last follow-up.
Forty-five of 51 patients were included in the comparison of baseline and last follow-up IOP and number of glaucoma medications. There were no differences between baseline and last follow-up IOP (p=0.22) or medications (p=0.32) (Table 4). Twenty-six patients that had not undergone phacoemulsification were included in the BCVA analysis. There was no significant difference in logMAR BCVA at last follow-up compared to baseline (p=0.07) (Table 4).
Postoperative complications and additional treatment
Two (3.9%) eyes experienced an acute angle-closure attack during follow-up. Angle-closure attacks occurred 222 and 510 days after ALPI, respectively. Both were treated pharmacologically and with repeat LPIs, and one required eventual cataract extraction. Two (3.9%) eyes underwent a repeat ALPI (one of which required eventual cataract extraction as well), and 1 (1.9%) eye underwent Ahmed valve insertion. Phacoemulsification was not performed with the Ahmed procedure in this eye because the patient was young without a visually significant cataract and had poor visual potential.
A total of 25 phacoemulsification procedures with or without adjunctive IOP-lowering measures were performed. Four (8%) were to remove a worsening visually significant cataract. The vision in these patients at the last visit before phacoemulsification was 0.34 (± 0.24) and the average IOP was 18 (± 4.5) mmHg. Twenty-one (41%) phacoemulsification procedures were performed to treat persistent angle dysfunction. The average vision of these patients at the last visit before phacoemulsification was 0.20 (± 0.33) and the average IOP was 17.8 (± 4.7) mmHg. Sixteen (31%) procedures were phacoemulsification alone, 2 (4%) had concomitant endoscopic cyclophotocoagulation (ECP), 2 (4%) had trabeculectomy, and 1 (2%) had ECP and goniotomy. The mean time to cataract surgery with or without adjunctive IOP-lowering measures after ALPI was 934 (± 694) days (approximately 31.1 months). One (1.9%) patient developed new PAS after ALPI. No other postlaser complications were observed.
Only a handful of studies have reported results of ALPI, and to the best of our knowledge the current study is the only to report survival data. Our results suggest that ALPI is a relatively safe and potentially effective treatment option for the management of PIS. Based on our clinical definition of procedure success the cumulative probability of success was 73% after 12 months and 58% after 24 months. Twenty-one patients required cataract extraction to treat a persistently narrow angle on average 934 (± 694) days (approximately 31.1 months) after ALPI. This suggests that ALPI may delay the need for cataract surgery to treat the narrow angle in patients without visually significant cataracts up to more than 2 years post-ALPI. We did not find any baseline characteristics including sex, non-white race, age, baseline IOP, medications, BCVA, or glaucoma status to be significant predictors of ALPI failure.
In the Kaplan-Meier survival analysis, increases in IOP ≥ 20% from baseline accounted for 7 (7/29, 24%) failure events. We did not observe any statistically significant changes in IOP at 12- months or last follow-up compared to baseline. This is similar to the findings reported by Romito et al. and Ritch et al., who did not observe any changes in IOP over the courses of their respective studies [6,7]. In contrast, Peterson et al. observed statistically significant reductions in IOP compared to baseline up to 4 years following ALPI [8]. However, the authors noted that at last follow-up or the last visit prior to additional treatment, the mean IOP gradually increased to the baseline level.
Different findings with regard to IOP reduction may be attributed to different patient populations, as the mean pre- ALPI IOP in Peterson’s study was 21.3 mmHg, while that of our study was 16.1 mmHg. Interestingly, Peterson et al. also reported that 36% of eyes underwent filtration surgery over the course of 4 years of follow-up. In contrast, only 4% of patients in the current study underwent subsequent filtration surgery. This difference could in part be attributable to slight differences in follow-up time, and the fact that patients in the Peterson study underwent trabeculectomy if IOP > 21 mmHg and if they were intolerant to topical therapy or noncompliant. In our study the decision to proceed with glaucoma filtration surgery remained subjective according to each respective surgeon.
The majority of failure events (13/29, 45%) that occurred in the Kaplan-Meier survival analysis were attributed to phacoemulsification to treat persistent angle dysfunction. In their study of 22 eyes with PIS, Peterson et al. reported that 41% of eyes required phacoemulsification on average 49 months after ALPI to treat a persistently narrow angle [8]. Likewise, 41% of eyes in the current study underwent eventual phacoemulsification, approximately 31.1 months after ALPI. With regard to additional IOP-lowering surgery, only 2 (4%) of eyes in the current study required filtering surgery, while 2 (4%) had ECP and 1 (2%) had ECP with angle surgery. This finding aligns with those of Romito et al. and Ritch et al., who did not report any instances of filtration surgery post-ALPI [6,7].
A substantial proportion (41%) of patients in our study underwent eventual phacoemulsification to treat progressive angle dysfunction. A prospective, randomized study comparing ALPI versus phacoemulsification after LPI could help determine the best clinical management algorithm for PIS. However, it remains that most PIS patients are young and have yet to develop cataract symptoms [9]. As such, low rates of post-laser complications and its ability to maintain IOP and medication burden, may suggest a role for ALPI in postponing the need for cataract surgery in these often-young patients in need of angleclosure prevention.
There are several limitations to our retrospective study. Our sample size is modest and therefore caution must be taken when extrapolating these findings to the population at large. Additionally, we cannot discount the possibility of underestimating failures based on angle configuration because gonioscopy was not performed regularly after the first post-ALPI visit. However, this is unlikely because our randomly sampled subset of patients underwent gonioscopy a median number of 4 times over 48 months, or once a year. Moreover, a more in-depth analysis of angle configuration following ALPI based on angle depth was prohibited by variable physician reporting of gonioscopy exam, and while the gonioscopy findings were carefully described on a case-by-case basis, they remain subjective. The decision to perform further interventions (i.e., repeat ALPI, phacoemulsification, filtration surgery) was based on the clinical judgement of the acceptable IOP for each individual case and not by predetermined study criteria. Lastly, this study would benefit from a comparative arm of patients with persistently narrow angles who did not receive ALPI after LPI. This was unfortunately impossible to execute as the standard of care for most providers at our institution as of 2019 is to perform ALPI in the setting of persistent appositional angle closure after LPI.
In summary, our results suggest that ALPI is an effective treatment option for PIS eyes with persistently narrow angles post-LPI. This may be especially relevant to young PIS patients without visually significant cataracts. However, it must be noted that the effect of ALPI may wane overtime. A substantial proportion of patients in the current study required further surgical intervention, most commonly phacoemulsification, to control IOP due to persistent angle dysfunction approximately 31.1 months after ALPI.
D.S.V and E.K. contributed to the conception and design of this work. E.K., M.C., and A.N. contributed to the collection of the data. E.K. and N.H. were involved in the analysis and interpretation of the results. E.K. drafted the manuscript, and D.S.V and T.C. provided critical revision of the article. All authors gave explicit approval of the final version to be published.
We are grateful to Mr. and Mrs. Charles and Anne Gifford, as well as Mr. Stephen Traynor, for their generous donations that supported this research.
Citation: Klug E, Chachanidze M, Nirappel A, Hall N, Chang TC, Solá-Del Valle D (2020) Effectiveness of Argon Laser Peripheral Iridoplasty in Plateau Iris Syndrome Patients after Laser Peripheral Iridotomy. J Clin Exp Ophthalmol. 11:848. DOI: 10.35248/2155-9570.20.11.848
Received: 29-May-2020 Accepted: 12-Jun-2020 Published: 19-Jun-2020 , DOI: 10.35248/2155-9570.20.11.848
Copyright: © 2020 Klug E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.