Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2020)Volume 9, Issue 3
The investigation on effect of mechanical, chemical, growth hormone and biofertilizer treatments on seed quality enhancement in Ashwagandha (Withania somnifera Dunal) was conducted at Department of Seed Science and Technology Laboratory, B. A. College of Agriculture, AAU, Anand during 2017-18. The experiment was laid out in Completely Randomized Design (Factorial) with two varieties, viz., JA 134 and GAA 1. Seeds of both the varieties were treated with different scarification, growth hormones and biofertilizer treatments and their combined effect on germination and other seed quality attributes was studied. The effect of pre-sowing seed treatment with growth hormone GA3 (200 ppm), bio-fertilizer treatment Phosphorous Solubilizing Bacteria and varieties showed significant effect on germination per cent, seedling length and seedling vigour indices of aswagandha. The maximum germination per cent was attained by the GAA 1 compared to JA 134.
Scarification; Hormone; Biofertilizer; Seedling; Vigour
In the changing world scenario, cultivation of medicinal plants has assumed greater importance due to the tremendous potential they offer in formulating newer drugs against many diseases and illnesses that affect human kind. India is officially identified for more than 3000 plants for their medicinal value and is recognized as one of the 12 mega diversity centers in the world. It ranks 10th in the world and 4th in Asia having 15 to 20 thousand plant species with medicinal value of which 30 per cent are considered as endemic to India [1].
Ashwagandha (Withania somnifera (L.) Dunal) belonging to Solanaceae family is a small woody shrub or herb that grows to usually 30 to 50 cm height (maximum of 150 cm). It is an adoptogenic herb and its roots, seeds and leaves are used in ayurvedic and unani medicines. It is cultivated over an area of 10,780 ha with a production of 8,429 tonnes in India. Based on the current trend the demand of ashwagandha would be around 12500 tonnes [2]. It is cultivated in many states of India such as Madhya Pradesh, Gujarat, Haryana, Maharashtra, Punjab, Rajasthan and Uttar Pradesh.
The seed germination is the first and foremost prerequisite in assessing the quality and optimizing yields from a seed lot. International Seed Testing Association (ISTA) has been recommending and prescribing the seed testing protocols for different species (i.e., requirements of temperature and substratum regularly). The long-period of seed dormancy possess problem in cultivating a species when immediate sowing and/or seed testing is required. Seed treatment is one of the methods adopted for quality seed production as it not only reduces the deleterious effects of damage to seed viability and vigour but also provides better avenues for their establishment, growth and development of seedlings. Some pre-sowing treatments are reported to be effective in optimizing germination in seeds of various medicinal plant species [3,4], therefore application of pre-sowing treatments (both physical and chemical) also need to be investigated in ashwagandha. Possibility of application of biofertilizers for producing quality seedlings in medicinal plants needs to be explored.
Germination of the seed is the initial and most crucial step in the propagation or laboratory testing of seed for certification. As the germination of the ashwagandha seed is irregular and takes more than 15 days, so there is urgent need to study on germination aspect of this crop. In order to have better germination and seedling quality parameters of ashwagandha, it is necessary to conduct germination treatments for breaking the dormancy in the laboratory conditions. The irregular and low germination is the main problem in the propagation of many medicinal plants which can be enhanced by application of biofertilizers [5]. The present experiment was conducted to study the effect of different scarification methods and different growth hormones, chemical treatments and biofertilizer treatments on seed germination, quality enhancement and other quality parameters in ashwagandha.
Experimental site and seed source
The laboratory experiment was conducted at Department of Seed Science and Technology, BACA, AAU, Anand. Freshly harvested seeds of ashwagandha variety JA 134 (V1: Jawahar Ashgand 134) was obtained from ICAR- Directorate of Medicinal and Aromatic Plant Research, Boriavi, Anand. Variety GAA 1 (V2: Gujarat Anand Ashwagandha 1) was obtained from Medicinal and Aromatic Plant Research Station, Anand Agricultural University, Anand.
Scarification
Mechanical scarification was done by taking five gram seeds at random from the seed lot of each variety and scarified in electrical seed scarifier for 1 minute and 2 minutes separately. For acid scarification, the seed was soaked in H2SO4 at both 25% and 50% concentration for 1 minute and 2 minutes. Later the seeds were washed under running tap water and were dried under shade. The untreated seeds were considered as control and it was taken from fresh seed lot.
For hot water treatment, seeds were being placed in muslin cloth bag and dipped in boiling water (100°C) and allowed to stand for 1 minute then removed and cooled at room temperature (25°C - 30°C). The same treatment was repeated for 2 minute. The different treatment combinations were S1: Control; S2: Mechanical Scarification (1 minute); S3: Mechanical Scarification (2 minute); S4: H2SO4 (25%) 1 minute; S5: H2SO4 (25%) 2 minute; S6: H2SO4 (50%) 1 minute; S7: H2SO4 (50%) 2 minute; S8: Hot Water (100°C) 1 minute; S9: Hot Water (100°C) 2 minute.
Seed treatment with different growth hormones and chemicals
Five gram seed were soaked for 24 hours in various growth hormones and chemical solution viz., GA3 (100 and 200 ppm), IAA (100 and 200 ppm) and KNO3 (0.5 and 1.0%) solution after that seeds were shade dried back to original moisture and then germination test was conducted.
For preparation of 100 ppm GA3 solution, 10 mg GA3 powder was weighted and dissolved in 3-5 mL of alcohol then after 100 mL volume was made up by adding distilled water. For 200 ppm solution 20 mg GA3 powder was taken and then after same procedure was repeated. For preparation of 100 ppm IAA solution, 10 mg IAA powder was weighted and dissolved in 3-5 mL of 1N NaOH solution then after 100 mL volume was made up by adding distilled water. For 200 ppm solution 20 mg IAA powder was taken and then after same procedure was repeated. For 0.5% solution of KNO3 0.5 gram of KNO3 powder was dissolved in 100 mL of water. For 1% KNO3 solution 1 gram of KNO3 powder was dissolved in 100 mL water. The different treatment combinations were G1: Control; G2: GA3 100 ppm; G3: GA3 200 ppm; G4: IAA 100 ppm; G5: IAA 200 ppm; G6: KNO3 0.5%; G7: KNO3 1.0%.
Seed treatment with different biofertilizers
Seeds were treated with biofertilizer cultures as per the recommended dose for seed treatment @5 mL/kg seeds diluted with required quantity of water and were soaked in liquid biofertilizers culture for 2-3 hrs and thereafter germination test was conducted. The different treatment combinations were B1: Control; B2: Azotobacter (Azotobactrer chroococcum); B3: Azospirillium (Azospirillium lipoferum); B4: PSB (Bacillus sp.); B5: Bio Potash (Enterobacter asburiae); B6: Bio NP (Azospirillium lipoferum + B. coagulans); B7: Bio NPK (Azotobacter + Azospirillium + Bacillus sp).
Germination %
The germination test was conducted by “Top of Paper (TP)” method. Seeds in four replications was taken at random from the fresh seed lot of each treatment and placed uniformly on germination paper in petri plate and kept in germinator. The temperature was maintained at 25 ± 0.5°C and the relative humidity at 95 ± 1 per cent. The final count of germination per cent was recorded on 14th day of germination test.
Seedling length (mm)
Ten normal seedlings were selected at random from the germination test. The length between the collar region and the tip of the primary shoot was measured as shoot length (mm). The length between the collar region and the tip of primary root was measured as root length (mm). The total seedling length was computed by using the following formula,
Seedling length (mm) = Shoot length (mm) + Root length (mm)
Seedling dry weight (mg)
Fresh weighed seedlings were placed at 80°C in hot air oven for 24 hrs for drying. Thereafter, the seedlings were removed and cooled in desiccator before weighing on an electronic balance. The weight of the dried seedlings was recorded and it was calculated and expressed in milligram (mg).
Seedling vigour indices
The seedling vigour indices were calculated by using the procedure suggested by Abdul- Baki and Anderson (1973) and expressed in whole number [6].
Seedling vigour index - I = Germination (%) × Seedling length (mm)
Seedling vigour index - II = Germination (%) × Seedling dry weight (mg)
The effect of different scarification methods on seed germination and other quality parameters
The germination per cent and its attributing characters were significantly influenced by varieties, scarification and their interactions (Figure 1). The highest mean germination per cent (72.77%), seedling length (39.63 mm), seedling vigour index-I (3224), vigour index-II (268.9) was observed in variety GAA 1 for mechanical scarification of 2 minutes among all the other treatments. The highest seedling dry weight was recorded with mechanical scarification for 2 minutes (6.78 mg) which was significantly superior among all the other treatments. The result from interaction of variety and treatment was found not significant.
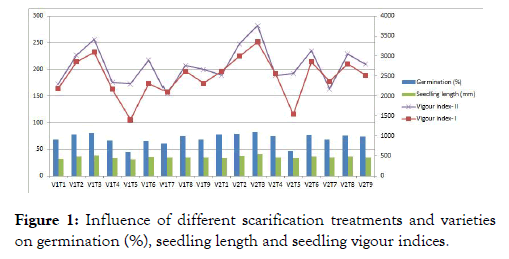
Figure 1: Influence of different scarification treatments and varieties on germination (%), seedling length and seedling vigour indices.
The effect of different growth hormone and chemical treatments on seed quality enhancement
The germination per cent and its attributing characters were significantly influenced by varieties, different growth hormones, chemical treatments and their interactions (Figure 2). The best result from interaction of varieties and treatment was observed for germination in GAA 1 with GA3 200 ppm (90.00%) and the lowest in control of variety JA 134 (68.00%). The highest seedling vigour index-I (4588), seedling vigour index-II (340.7) were recorded compared to all other treatments. The highest seedling dry weight (6.63 mg) was recorded in GA3 200 ppm and the lowest in KNO3 0.5% (5.48 mg).
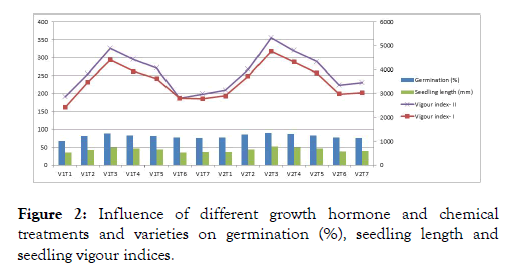
Figure 2: Influence of different growth hormone and chemical treatments and varieties on germination (%), seedling length and seedling vigour indices.
The effect of different biofertilizer treatments on seed quality enhancement
The germination per cent and its attributing characters were significantly influenced by varieties, different biofertilizer treatments and their interactions (Figure 3). The seed treatment Phosphorous Solubilizing Bacteria recorded with highest mean germination (90.00%), seedling length (50.03 mm), seedling vigour index-I (4520), vigour index-II (619.1) which was significantly superior among all the other treatments. The seedling dry weight was recorded highest in Phosphorous Solubilizing Bacteria (6.85 mg) and the lowest results were observed in control (5.33 mg) which was at par with Azotobacter (5.51 mg) (Figure 4). However the interactions between biofertilizer treatments and varieties were found non-significant.
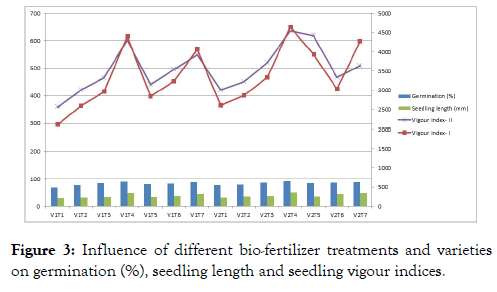
Figure 3: Influence of different bio-fertilizer treatments and varieties on germination (%), seedling length and seedling vigour indices.
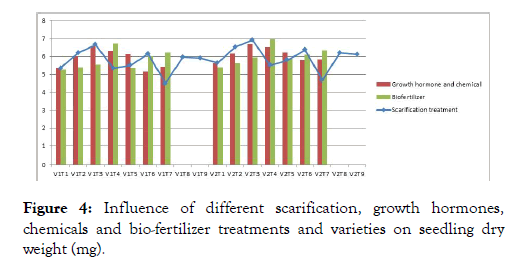
Figure 4: Influence of different scarification, growth hormones, chemicals and bio-fertilizer treatments and varieties on seedling dry weight (mg).
It is evident from the study that mechanical and acid scarification improved germination of seeds indicated that the seed dormancy is due to the presence of hard seed coat. Mechanical scarification treatments caused wear on seed coat without damaging the embryo, allowing water to enter in an amount sufficient for seedling emergence which culminate with the supply of energy and nutrients necessary to resume embryo axis growth and for germination [7,8]. The enzymatic and hormonal mechanism stimulates metabolic process such as sugar mobilization, protein hydrolysis, oxidation etc., which leads to increase in seedling fresh weight and seedling dry weight [9]. The mechanical scarification of the seeds of Parkia biglobosa may be effective for breaking seed coat dormancy and improving the seedling vigour by increased germination percentage [10].
The germination improvement by KNO3 and GA3 treatment might be due to the activity of KNO3 and GA3 as a-denovo synthesis and helps in dormancy breaking action thereby stimulating metabolic processes such as sugar mobilization, protein hydrolysis, oxidation etc., [11]. Priming of seeds with inorganic salts like KNO3 and GA3 may alter enzyme activity which has direct or indirect effects on seed germination and seedling growth and development [12,13]. The higher seedling vigour indices are due to hormonal treatments is the beneficial role of the elements in cell division and cell elongation [14].
The role of biofertilizers Azospirillum and phosphobacteria in enhancing the availability of nitrogen and phosphorus and other nutrients with consequent enhancement in the metabolic activity resulted in higher germination [15,16]. An increase in the seedling growth in seeds treated with biofertilizers might be due to the increased cytokinin production which triggers the production of growth regulating substances like auxin, GA and cytokinin which promotes cell division [17]. The PSB (Bacillus sp.) recorded the highest seedling vigour indices may be due to the beneficial role of the elements in cell division and cell elongation [18].
The study can be concluded that the mechanical scarification for two minutes recorded highest germination percent and seedling parameters compared to control. The maximum germination per cent was attained by the growth hormone GA3 200 ppm. Also the biofertilizer Phosphorous Solubilizing Bacteria culture recorded the highest seedling germination and its attributing parameters followed by Bio NPK in the variety GAA-1.
The work has been carried out at Department of Seed Science and Technology, BACA, AAU, Anand. The authors express their sincere thanks and gratitude to Anand Agricultural University for extending research facilities for conducting the present study.
Citation: Sapra NC, Kalyanrao P, Sasidharan N, Das A, Susmitha P (2020) Effect of Mechanical, Chemical, Growth Hormone and Biofertilizer Treatments on Seed Quality Enhancement in Ashwagandha (Withania somnifera Dunal) Med Aromat Plants (Los Angeles) 9: 350. doi: 10.35248/2167-0412.20.9.350.
Received: 19-Oct-2019 Accepted: 22-May-2020 Published: 30-May-2020 , DOI: 10.35248/2167-0412.20.9.350
Copyright: © 2020 Sapra NC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.