Clinical Pediatrics: Open Access
Open Access
ISSN: 2572-0775
ISSN: 2572-0775
Research Article - (2024)Volume 9, Issue 1
Background: Fever is the most common cause for neonates visiting the Emergency Room (ER). Because bacterial versus viral infection symptoms exhibited by neonates are similar, clinical assessment and history taking are usually insufficient to distinguish etiology, even for experienced doctors. This clinical uncertainty drives antibiotic misuse, unwarranted invasive diagnostic procedures and longer hospitalizations.
Methods: A prospective study conducted at Hillel Yaffe Medical Center from 2019 to 2021 to investigate the expression of three serum proteins: Tumor Necrosis Factor-Related Apoptosis Inducing Ligand (TNF-TRAIL), interferon gamma-Induced Protein-10 (IP-10) and C-Reactive Protein (CRP) in neonates up to three months of age with microbiologically confirmed bacterial infections versus viral infection.
Results: Out of 107 infants, 94 had sufficient serum for measuring the biomarkers. Of these, 85 had febrile illness, of which 12 had microbiologically confirmed bacterial disease and 73 viral diseases. Mean age for both groups was 44 days old. Neonates with bacterial infections exhibited lower levels of TRAIL (p<0.05) and IP-10 (p=0.18) and higher levels of CRP (p<0.05). IP-10 levels > 1100 pg/ml were only observed in non-bacterial infected neonates.
Conclusions: There are significant differences in IP-10, TRAIL and CRP expression levels in response to bacterial versus viral infection in neonates. A prognosis based on these biomarkers can potentially improve the management of neonates with fever, by aiding in the differentiation between bacterial and viral disease, thereby reducing unnecessary work-ups and treatments.
Neonate; Antibiotic; C-Reactive Protein (CRP); Receiver Operating Characteristic (ROC) model; TNF- Related Apoptosis Inducing Ligand (TRAIL)
Fever is the predominant reason for pediatric emergency department visits, with a considerable number of cases involving infants up to three months of age [1]. Diagnosing and treating infants this age presents a significant challenge to clinicians, as the clinical manifestations in neonates, which aid in distinguishing between serious bacterial infections and mild viral illnesses, are often unreliable. Consequently, there is considerable variation in the investigative and treatment approaches employed worldwide for febrile infants up to three months of age [1,2].
The clinical criteria utilized for categorizing neonates according to disease severity and determining treatment (Rochester, Philadelphia) are based on a combination of patient history, physical examination findings and laboratory results, some of which necessitate invasive procedures such as lumbar puncture or bladder puncture [1]. While these criteria possess a high negative predictive value for identifying or excluding serious bacterial infections, they demonstrate low specificity and low positive predictive values, leading to potentially unnecessary antibiotic treatments and extended hospital stays [1].
Antibiotics are of the greatest discoveries in medicine and are among the most life-saving medical interventions. Their efficacy and presumed safety have led to their widespread use, which sometimes is irrational or even inappropriate. In infants and children, antibiotics are among the most used drugs [3].
The first three months of life, during which the initial gut microbiota is acquired, is a critical phase. Healthy microbiome development can be interrupted by external perturbations, like antibiotics. While the use of perinatal broad-spectrum antibiotics has become common in modern obstetric and neonatal practice, increasing evidence shows that exposure to antibiotics early in life is associated with profound effects on the gut microbiome and various disorders later in life like atopy, Inflammatory Bowel Disease (IBD), diabetes and obesity [4]. A recent prospective study describes long-term differences in gut microbiome composition of new-born infants who were exposed to antibiotics, as well as the emergence of antibacterial resistance genes [5].
Despite minimal changes to the original criteria since their inception, several inflammation markers have been proposed in recent years to assist in predicting the risk of serious bacterial infection in neonates and infants up to three months of age. The primary markers include C-Reactive Protein (CRP) and procalcitonin [1].
CRP has been assessed in multiple studies involving febrile neonates up to three months of age, with specific values demonstrating higher positive and negative predictive values than White Blood Cell (WBC) in predicting serious bacterial infections. In a retrospective study conducted by Olaciregui, et al., [1] encompassing 347 infants up to three months of age, CRP was identified as a superior laboratory predictive index compared to WBC for suspected serious bacterial infections. Moreover, a prospective study conducted in Israel, involving 892 infants up to three months of age, demonstrated that a CRP value exceeding 2 mg/dl yielded higher sensitivity, specificity, positive predictive value and negative predictive value than traditional WBC thresholds for assessing the risk of serious bacterial infections (values above 15,000 or below 5,000) [6]. This study also revealed that 50% of infants with a CRP value above 5 mg/dl presented with a serious bacterial infection. Procalcitonin, has demonstrated equivalent predictive capabilities to CRP in a limited number of studies for forecasting serious bacterial infections in neonates [1].
There is a pressing clinical need to develop indicators that facilitate the identification or exclusion of bacterial infections in this vulnerable infant population, to minimize unnecessary antibiotic treatment, reduce hospital stays and limit invasive diagnostic procedures. One recent approach to differentiate between bacterial and viral etiology in febrile infants involves examining the immune system response. A signature based on the levels of three soluble blood proteins in infants (TRAIL, IP-10 and CRP) has been studied, revealing over 90% accuracy in discriminating between bacterial and viral infections in infants aged three months and older. This method has shown that relying on a combination of multiple proteins overcomes the limitations of using a single marker [7,8]. Although this data shows great promise, it did not include neonates under three months of age. Therefore, we were interested in knowing whether these three biomarkers are also differentially expressed in this age group.
A prospective, non-interventional study was carried out at Hillel Yaffe Medical Center between 2019 and 2021, enrolling neonates under three months of age presenting with acute febrile illness. Inclusion criteria included age up to three months (inclusive), fever above 38°C within 24 hours prior to emergency department arrival and the necessity for blood tests to identify the infection source, as determined by the attending physician. Exclusion criteria included gestational age below 37 weeks, ongoing antibiotic treatment, perinatal antibiotic administration, congenital or acquired immunosuppression, known Human Immuno Deficiency Virus (HIV) or hepatitis B/C infection, acute infectious disease within two weeks before enrollment, prior hospitalization and extended hospital stay during birth, unexplained hyperbilirubinemia and metabolic or other chronic diseases.
Blood tests, blood cultures, urine tests and additional diagnostics (e.g. chest X-rays, lumbar punctures) were performed based on the treating physician's discretion. Demographic data and medical history were collected for each participant. Blood samples were separated into respective fractions, residual separated samples were stored at -70°C within two hours of separation and subsequently analyzed for the protein panel comprising TRAIL, IP-10 and CRP using immunoxpert and MeMed BV (MeMed diagnostics, Tirat HaCarmel, Israel).
Statistical analysis
Descriptive statistics, including means, standard deviations, medians, percentiles and ranges, were calculated for all variables in the study. The dependent variable in this investigation is the fever source as a nominal variable, either an acute infectious fever source or a mild viral disease. The normality assumption for continuous variables (e.g. neonatal age, temperature, hours since symptom onset, birth week, birth weight and laboratory tests) were assessed using the kolmogorov-smirnov test. Depending on the test results, differences between the study groups were evaluated using either the t-test or the Mann- Whitney U test. Categorical variables (e.g. lumbar puncture, mode of birth, postnatal complications) were analysed using the fisher's exact test. To distinguish between febrile illness due to acute bacterial infection and mild viral illness, the Receiver Operating Characteristic (ROC) model was employed to compare WBC, ANC and CRP levels and the Area Under the Curve (AUC) was calculated. A multivariate logistic regression model was constructed to predict acute bacterial infection based on independent variables. Statistical significance was established at p<0.05.
Clinical findings
A total of 107 samples were collected from neonates up to three months old who visited the pediatric emergency department. Out of these, 94 (88%) tubes contained sufficient serum for protein analysis in the laboratory. Among these neonates, 85 (90%) presented with acute febrile illness during sample collection. Out of all the neonates, 12 (13%) had confirmed bacterial infections through blood cultures, urine cultures, Cerebro Spinal Fluid (CSF) cultures or stool cultures. The remaining 73 (87%) infants were adjudicated to a viral infection, of which 33 (45%) had a positive viral detection using a Polymerase Chain Reaction (PCR)-based detection panel. The most common viruses detected were enterovirus 14 (42%), Respiratory Syncytial Virus (RSV) 7 (21%), adenovirus 2 (6%), parainfluenza 2 (6%) and Corona Virus Disease-19 (COVID-19) 3 (9%). Some of the panels were found to be positive for more than one virus: RSV+adenovirus 3 (9%), Human Meta Pneumo Virus (HMPV)+parainfluenza 1 (3%), RSV+parainfluenza 1 (3%) (Table 1).
| Virus | Absolute Number | Percentage (%) |
|---|---|---|
| Enterovirus | 14 | 43 |
| RSV | 7 | 21 |
| COVID 19 | 3 | 9 |
| Parainfluenza | 2 | 6 |
| Adenovirus | 2 | 6 |
| RSV + Adenovirus | 3 | 9 |
| RSV + Parainfluenza | 1 | 3 |
| HMPV + Parainfluenza | 1 | 3 |
Note: HMPV: Human Meta Pneumo Virus; RSV: Respiratory Syncytial Virus; COVID 19: Corona Virus Disease of 2019
Table 1: The most common viruses were described.
Clinical characteristics
The clinical characteristics of the patients, subdivided into total patients, bacterial and viral population, are described in (Table 2). There were no significant differences in patient age (46.5, 41 days; p=0.96), date of birth (38, 39 weeks; p=0.10), maximal temperature (38.5, 38.5°Celsius; p=0.38), time from symptoms (6, 6 hours; p=0.77), WBC (11.4, 10.3; p=0.29) and Absolute Neutrophil Count (ANC) (5.6, 3.3; p=0.09).
| Bacterial group | Viral group | P-value | |
|---|---|---|---|
| Age (days) | 46.5 | 41 | 0.96 |
| Gestational age (weeks) | 38 | 39 | 0.1 |
| Maximal temperature (°C) | 38.5 | 38.5 | 0.38 |
| Time from symptoms (hours) | 6 | 6 | 0.77 |
| WBC (X103) | 11.4 | 10.3 | 0.29 |
| ANC (X103) | 5.6 | 3.3 | 0.09 |
| Hospitalization duration (days) | 4 | 3 | <0.05 |
| LP performed (%) | 91.7 | 47 | <0.05 |
| Treated with Abx (%) | 100 | 47 | <0.05 |
Note: ANC: Absolute Neutrophil Count; WBC: White Blood Cell; LP: Lumbar Puncture; Abx: Antibiotics.
Table 2: Clinical characteristics of the patients are subdivided into total patients and bacterial and viral population are described.
A statistically significant difference was observed in the number of neonates who received antibiotic treatment favouring those with bacterial infections (100, 47 percentage; p<0.05), also in the number of lumbar punctures performed (91.7, 47 percentage; p<0.05) and in the hospitalization duration (4, 3 days; p<0.05).
IP-10, TRAIL and CRP proteins
Differential expression of the three host protein biomarkers was assessed for 94 (88%) of the patients of which residual serum was stored. All three proteins were found to be differentially expressed when comparing the bacterial and viral patient population: CRP (86 mg/l vs. 13 mg/l; p<0.001); TRAIL (81 pg /ml vs. 183 pg/ml; p<0.001) and IP-10 (338 pg/ml vs. 1331 pg/ml; p=0.18) (Figure 1).
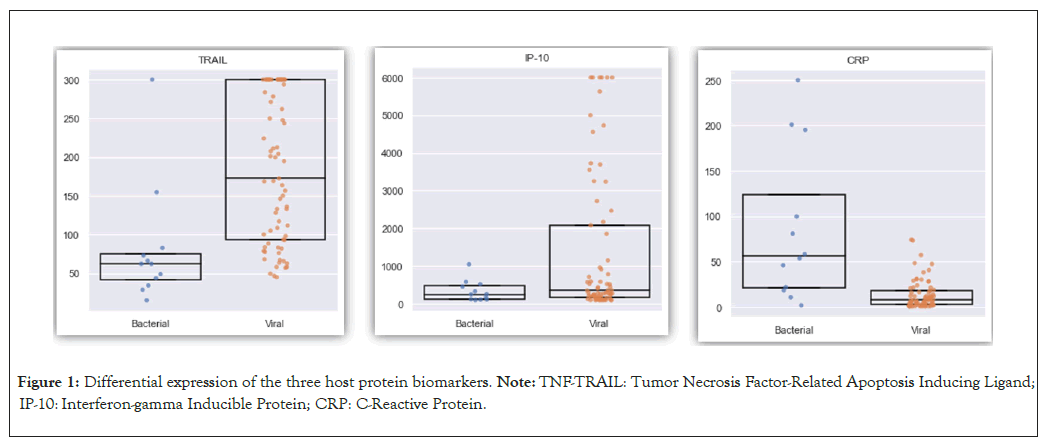
Figure 1: Differential expression of the three host protein biomarkers. Note: TNF-TRAIL: Tumor Necrosis Factor-Related Apoptosis Inducing Ligand; IP-10: Interferon-gamma Inducible Protein; CRP: C-Reactive Protein.
Neonates aged 0-28
We subdivided our neonates into two age groups and investigated whether there is a difference in protein expression change between the two groups. The first group included neonates aged 0 to 28 days, consisting of 23 neonates (35%), four (17%) with bacterial infections and 19 (83) with viral infections. In this younger age group, the same changes in protein expression were observed between the two groups as previously: CRP (69 mg/l vs. 8 mg/l; p=0.08); TRAIL (48 pg/ml vs. 194 pg/ml; p=0.09) and IP-10 (288 pg/ml vs. 788 pg/ml; p=0.18).
In this younger age group, CRP levels increased more in bacterial infections compared to IP-10 and TRAIL levels, which were higher in viral infections (Figure 2).
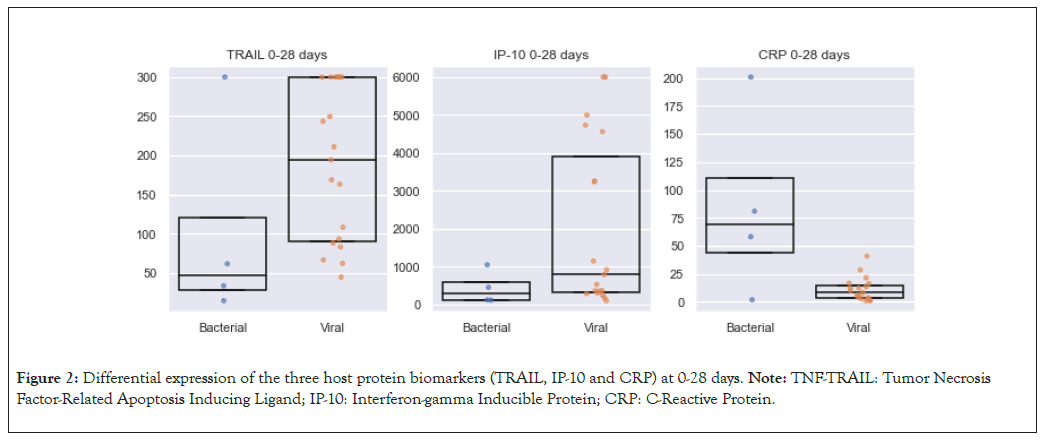
Figure 2: Differential expression of the three host protein biomarkers (TRAIL, IP-10 and CRP) at 0-28 days. Note: TNF-TRAIL: Tumor Necrosis Factor-Related Apoptosis Inducing Ligand; IP-10: Interferon-gamma Inducible Protein; CRP: C-Reactive Protein.
Neonates aged 29-60 days
The second group comprised of older neonates aged 29 to 60 days, with a total of 42 neonates (65%), four (9%) with bacterial infections and 38 (91%) with viral infections. In this group, the same results were observed in protein expression: CRP (120 mg/l vs. 6 mg/l; p=0.01); TRAIL (55 pg/ml vs. 202 pg/ml; p=0.003) and IP-10 (208 pg/ml vs. 350 pg/ml; p=0.28) (Figure 3). To assess whether any single biomarker could be used to rule out bacterial infection, we looked at a potential cut-off value to be used for one of the biomarkers. Using a cut-off value of 1100 pg/ml for IP-10, we found that it correctly classified all patients as viral above the threshold and bacterial below the threshold (i.e. IP- 10>1100 pg/ml has an NPV of 100%).
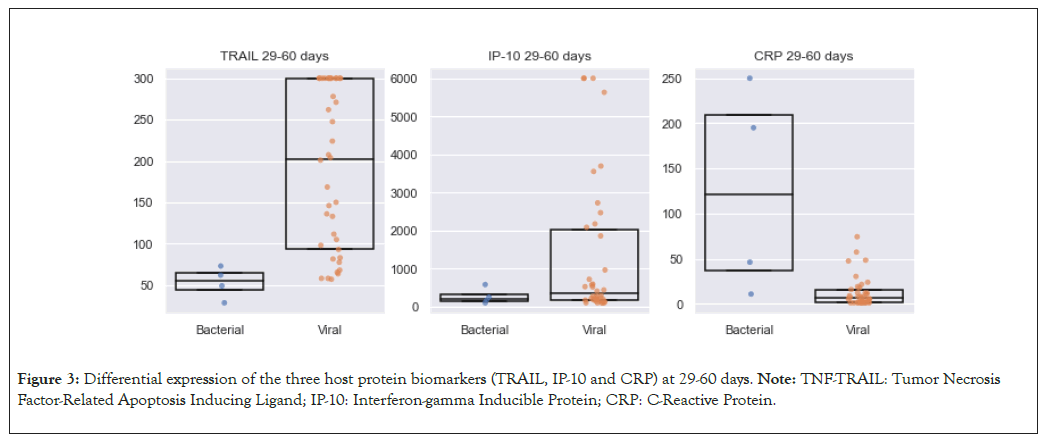
Figure 3: Differential expression of the three host protein biomarkers (TRAIL, IP-10 and CRP) at 29-60 days. Note: TNF-TRAIL: Tumor Necrosis Factor-Related Apoptosis Inducing Ligand; IP-10: Interferon-gamma Inducible Protein; CRP: C-Reactive Protein.
One of the most common reasons for neonates up to the age of three months to visit the pediatric ER is acute febrile illness. Clinical and laboratory criteria that have been used to differentiate between viral and bacterial diseases have changed over the years and are insufficient.
Fever is often the only sign of illness in small infants who appear well when they are admitted to the pediatric ER. This makes it clinically difficult to differentiate between neonates with a mild viral disease and those with a more severe bacterial disease that can lead to sepsis and death if left untreated. Due to this clinical uncertainty, many neonates with fever undergo invasive tests, administration of broad-spectrum antibiotics and are sometimes hospitalized due to a simple viral illness that does not require treatment [9].
The importance of the human gut microbiome in health and disease is becoming increasingly clear. Disturbances of the gut microbiota after birth are associated with a broad array of health problems in early infancy and later in life, such as infantile colic, wheezing, allergies, functional gastrointestinal disorders, obesity and generally an altered immune development [10]. These various causes of disturbances include Caesarean Section (CS) delivery, formula feeding (as opposed to breastfeeding) and antibiotics on the developing neonatal gut microbiome [10].
Although antibiotics confer significant health benefits in treating or preventing bacterial infections, more and more evidence illustrates their detrimental effect on host microbiota homeostasis, posing a serious menace to the global public health [11]. In recent years, it has become evident that infants who are subjected to frequent antibiotic exposures due to their vulnerability to infection reflect increased susceptibility to a wide spectrum of diseases, including infections in later life [11].
Certain serum proteins were found when searching for markers which are differentially expressed in viral versus bacterial infections [12]. In previous studies in infants over three months of age, it was found that TRAIL and IP-10 proteins increase more in viral diseases compared to bacterial diseases and that CRP increases more in bacterial diseases compared to viral diseases.
These differences have never been tested in infants under the age of three months and our study, to the best of our knowledge, is the first to test these same serum proteins in this population. Our results show that, like in older neonates, IP-10 and TRAIL also increase significantly in viral diseases compared to bacterial diseases in younger neonates, which can be used as an additional diagnostic tool in the management of neonates under three months of age with fever in the ER.
Neonates who appear to be toxic should undergo a full investigation, including invasive tests and the administration of broad-spectrum antibiotic treatment, according to clinical guidelines. In neonates under the age of 28 days whose physical examination is normal, a full sepsis routine should be required because of the high risk of dangerous bacterial infection [13]. However, there is still disagreement regarding the need for invasive tests and the administration of broad- spectrum antibiotic treatment for neonates between the ages of 29-60 days, when representing with an acute febrile illness and their physical examination is normal without risk factors for bacterial infection.
In our study, we showed that even when testing for the same proteins in different age groups, between the ages of 0-28 days and between the ages of 29-60 days, similar results were found. IP-10 and TRAIL proteins increased more in neonates with viral diseases compared to those with bacterial ones. These findings can be utilized as significant management approach tools for neonates in the pediatric ER.
In our study, we further evaluated the IP-10 protein which increased significantly in neonates with viral diseases. We found that 21 neonates had an IP-10 protein value of >1100 pg/ml, of which 66% of the neonates underwent a lumbar puncture and 62% received antibiotic treatment, when in practice 0% of those neonates had evidence of a bacterial disease. In other words, almost two thirds of the neonates underwent unnecessary invasive examination, antibiotic treatment and hospitalization. Conversely, none of the neonates with bacterial disease had IP-10 values above this value, so the (Negative Predictive Value) NPV of the IP-10 protein in our study above 1100 pg/ml is 100%. This threshold can prevent invasive procedures and treatment of neonates in this age group.
Different approaches to the management of fever in young infants have shown the need for an up-to-date, evidence-based guideline developed by a professional organization. In 2021, the American Academy of Pediatrics issued new guidelines to assist in the management of small infants with acute febrile illness who are admitted to pediatric intensive care units.
The primary emphasis of these guidelines is to categorize infants into age groups, like the approach we used in our study. The new guideline approach aims to minimize unnecessary invasive tests and treatments. It suggests that infants aged 22 days with a well appearance and no signs of inflammation in blood test, can be discharged home from the ER without the needs for a lumbar puncture or any antibiotic treatment [14].
We offer a new tool to caregivers in medical centers who treat neonates with acute febrile illness. A rapid IP-10 protein blood test can help the doctor decide if the neonate who looks well has a viral disease. This will consequently prevent the performance of unnecessary painful invasive tests, lower the incidence of providing unnecessary antibiotic treatment that can increase resistance in the future and prolong hospitalization in neonates.
The main limitations of the study are a small number of patients with a bacterial infection and considering this, a multicentre study should be conducted to examine if the IP-10 index above 1100 pg/ml is indeed a positive predictor for viral infection and a negative predictor for bacterial infectious disease in the age group of 29-60 days.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Zahalka D A (2024) Differential Expression of Tumor Necrosis Factor-Related Apoptosis Inducing Ligand (TNF-TRAIL) and Interferon- gamma Inducible Protein (IP-10) Biomarker Levels in Neonatal Infections: Potential Role in Ruling out Bacterial Infections to Promote Antibiotic Stewardship. Clin Pediatr. 09:257.
Received: 02-Jan-2024, Manuscript No. CPOA-24-28903; Editor assigned: 04-Jan-2024, Pre QC No. CPOA-24-28903 (PQ); Reviewed: 18-Jan-2024, QC No. CPOA-24-28903; Revised: 25-Jan-2024, Manuscript No. CPOA-24-28903 (R); Published: 01-Feb-2024 , DOI: 10.35248/2572-0775.24.09.257
Copyright: © 2024 Zahalka D A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.