Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2014) Volume 5, Issue 6
Objective: To evaluate the relationship between various cytokine levels in aqueous humor and diabetic macular edema (DME) types as determined by optical coherence tomographic (OCT) patterns.
Methods: The study examined 76 patients with DME at early phase. Ten patients who underwent cataract surgery served as controls. Eyes with DME were divided into four groups, based on OCT patterns of DME, prior to intravitreal bevacizumab injection (IVB). Patterns included sponge-like diffuse retinal thickening (SDRT) (n=27), cystoid macular edema (CME) (n=18), serous retinal detachment (SRD) (n=15), and combined CME and SRD (n=16). Visual acuity, central macular thickness (CMT) through OCT, and aqueous cytokine concentrations of interleukin (IL)-6, IL-8, IL-10, IL-13, monocyte chemo attractant protein-1 (MCP-1), vascular endothelial growth factor (VEGF) were measured in all subjects. The odds ratio (OR) between aqueous cytokines and each DME pattern were calculated using multivariate regression analysis.
Results: After IVB, CMT was reduced in all groups, but the highest reduction ratio occurred in eyes with SDRT and CME. Aqueous analyses showed that VEGF was associated with development of SDRT (P=0.003, OR=1.043, 95% CI=1.015-1.072). IL-6, MCP-1 were related with CME (P<0.001, OR=1.025, 95% CI=1.011-1.039; P<0.001, OR=1.003, 95% CI=1.001-1.004, respectively). IL-6 was also associated with SRD (P=0.045, OR=1.018, 95% CI=1.006-1.030). Furthermore, IL-6, VEGF were related with Combined pattern (P=0.031, OR=1.013, 95% CI=1.002-1.024; P=0.038, OR=1.014, 95% CI=1.014-1.043, respectively).
Conclusion: Specific aqueous cytokines may play an important role in developing each DME pattern based on OCT.
Keywords: Diabetic macular edema; Pattern; Aqueous; Cytokine; OCT
Diabetic macular edema (DME) is a major cause of central vision loss in patients with diabetic retinopathy. The pathophysiology of DME is thought to be multifactorial. Some authors suggested etiology of DME is the breakdown of the inner and/or outer retinal-blood barriers, which are located in the retinal capillary endothelial/retinal pigment epithelial (RPE) cell tight junctions [1,2]. It is well-known that vascular endothelial growth factor (VEGF) causes blood vessel hyper-permeability, and this is thought to be a main cause of DME [3]. Therefore, intravitreal bevacizumab (IVB) injection has been used to effectively treat DME [4,5].
However, there are numerous studies by many authors demonstrating no specific efficacy of IVB for chronic and/or refractory DME [6-8]. In some cases, IVB is less effective in treating DME than intravitreal triamcinolone acetonide (IVTA) [6,7]. This is thought to result from elevated levels of other inflammatory cytokines, as well as VEGF [3,6-8]. It is known that DME develops through multiple processes related to inflammation. Moreover, recent studies have shown that it has been found often the differences on the response for the treatment of IVB based on optical coherence tomography (OCT) patterns [9]. Also, it has been our clinical observation that IVB has different results for different DME types. It is considered that other inflammatory cytokines would have an important role in both the underlying pathogenesis of each DME type and the mechanism of intravitreal injections.
In this study, we attempted to determine whether aqueous cytokines can be related with the development of each DME based on OCT at early stage. This allowed us to better understand the pathogenic role of various cytokines in each type of DME and to determine the effective treatment method.
The study conducted adhered to the tenets of the Declaration of Helsinki, and informed consent was obtained from all subjects before performing any study procedure or examination. All surgeries were performed at the Kangdong Sacred Heart Hospital, and this study protocol was approved by the institutional review board of Kangdong Sacred Heart Hospital in Seoul, Korea.
Study population
This study was performed from January 2009 to January 2011. All cases had received one IVB injection and paracentesis with a full explanation under the agreement at the Department of Ophthalmology at Kangdong Sacred Heart Hospital to treat DME and had been followed for a minimum of 6 months following IVB therapy. Included eyes also met the following criteria: (1) central macular thickness (CMT) >250 µm, as measured on a spectral domain OCT (Cirrus HD-OCT; Humphrey Zeiss, Inc, San Leandro, CA) through macular cube scan mode, (2) clinically significant macular edema, as defined by the Early Treatment Diabetic Retinopathy Study (ETDRS) [10], (3) had experienced decreased vision within 1 month. Exclusions were previous vision loss, previous panretinal or focal laser photocoagulation, prior intraocular surgery or injection, presence of iris neovascularization, active proliferative diabetic retinopathy, significant media opacity, and any other macular diseases. Additionally, patients who had any ophthalmic and/or uncontrolled systemic disease (including cataract, glaucoma, poor glucose control, and uncontrolled systemic hypertension) that could have affected the study eye were excluded from this study. To compare results with normal eyes, 10 non-diabetic patients who had undergone cataract surgery during the same period were selected as controls.
Optical coherence tomography measurements and pattern determinations
The thickness of macular (central macular thickness, CMT) was measured by analyzing retinal thickness of a 0.5 mm radius circle centered on the macula. The OCT scans were acquired with the macular cube scan protocol. Horizontal and vertical line scans through the macula were also acquired (scan length 6 mm) in the standard, linear cross-hair pattern and were centered according to the corresponding red-free image to determine DME pattern. All preoperative OCT scans were classified into the 4 DME groups by three authors (J.Y.K., Y.J.J., S.P.P.) (Figure 1).
Figure 1: Different patterns of diabetic macular edema (DME), as represented on optical coherence tomography (OCT) images. The four types of DME were categorized as (A) diffuse retinal thickening (SDRT), which appeared as sponge-like retinal swelling, (B) cystoid macular edema (CME) which had intraretinal cystoid spaces, (C) serous retinal detachment (SRD) which had serous elevation of the retina with a clear space between the retina and the retinal pigment epithelium, and (D) combination DME, which presented as a combination of CME and SRD.
Cases in which three authors did not agree with types of DME were excluded. Cases in which it was difficult to define the pattern were excluded. Cases in which there were changes to other patterns during follow up periods were excluded. Eyes that had epiretinal membrane and/or vitreomacular traction were excluded. The study groups were defined as follows: simple cataract non-diabetic controls (Group 1, n=10 eyes), sponge-like diffuse retinal thickening (SDRT, Group 2, n=27 eyes), cystoid macular edema (CME, Group 3, n=18 eyes), serous retinal detachment (SRD, Group 4, n=15 eyes), and combined CME and SRD (Combined, Group 5, n=16 eyes) (Table 1).
| DME groups | P-value* | ||||||
|---|---|---|---|---|---|---|---|
| Control | SDRT | CME | SRD | Combined | All | ||
| Patients, n (eyes) | 10 | 27 | 18 | 15 | 16 | 76 | |
| Age (years) | 61.3±6.4 | 61.8±6.9 | 60.1±11.1 | 58.1±13.3 | 57.3±13.1 | 59.7±10.7 | 0.638 |
| Sex (male/female) | 4/6 | 9/11 | 7/10 | 10/12 | 8/9 | 34/42 | 0.789 |
| Diabetes Duration (years) | 8.3±2.4 | 7.3±1.6 | 7.9±2.6 | 9.4±2.8 | 8.2±2.4 | 0.079 | |
| Hypertension (yes/no) | 7/3 | 15/12 | 14/4 | 10/5 | 13/3 | 62/14 | 0.393 |
Table 1: Baseline clinical characteristics of the diabetic macular edema and control group.
Study design
All patients underwent a thorough ocular examination before and after IVB therapy, which included best-corrected visual acuity (BCVA) measured with a logarithm of the minimum angle of resolution (logMAR) chart, intraocular pressure, slit lamp examination, fundus examination, fluorescein angiography, and OCT. After 3 month of IVB therapy, BCVA and CMT were analyzed in each of the study groups. In addition, concentrations of interleukin (IL)-6, IL-8, IL-10, IL-13, monocyte chemoattractant protein (MCP)-1, and VEGF were measured in aqueous humor samples.
Intravitreal bevacizumab injection and aqueous humor collection
All IVB injections were performed in the operating room under sterile conditions. The ocular surface was first disinfected with 5% povidone iodine after topical anesthesia had been administered 2-3 drops proparacaine (Alcaine®, Alcon, Ft. Worth, TX). Intravitreal bevacizumab (1.25 mg/0.05 mL) injection was done using a 1 mL syringe with a sterile 30-gauge needle. The injection was done 3.5 mm from the corneal limbus through the pars plana. A 0.05 mL sample of aqueous humor was collected from each patient immediately after IVB injection. In the 10 control eyes (10 patients), an aqueous humor sample was obtained immediately prior to cataract surgery.
Statistical analyses
The traditional enzyme linked immunosorbent assay (ELISA) and Luminex multiplex bead assay showed excellent correlation and were performed using the milliplex human cytokine panel I-6 plex kit (xMAP; Luminex Corp. Austin, TX, USA) on the aqueous humor specimen [11]. All analyses were performed following the manufacturer’s testing procedures to examine 6 factors (IL-6, IL-8, IL-10, IL-13, MCP-1, and VEGF). The aqueous specimen was not diluted, and all procedures were done without light at room temperature. The specimen was registered into the suspension array system and compared to a standard curve for each related factor, created using manufacturer reference cytokine solutions. Every specimen was measured twice using the same procedure to ensure accuracy. The measurable cytokine concentration range was 1.0-10,000 pg/mL. All cytokine concentration results are presented as mean ± standard deviation, with the data range provided in parentheses (e.g., minimum-maximum value).
The Wilcoxon signed rank test was used to compare results before and after IVB therapy. The Kruskal-Wallis and Mann-Whitney tests were used to analyze data from multiple groups and were performed for comparative analyses. Univariate and multivariate binomial logistic regression was used to identify correlations between aqueous cytokine concentration and the development of each type of DME. Spearman’s correlation test was used verify correlations between cytokine factors and central macular thickness. All statistical analyses were performed using the SPSS statistical software package (SPSS 21.0 for Windows, SPSS Inc., Chicago, IL, USA). Statistical significance was defined as a P-value<0.05.
Seventy-six eyes with DME from 76 patients (34 men, 42 women) were enrolled in the DME group and 10 eyes from 10 control patients (4 men, 6 women) were enrolled in the control group. Baseline characteristics for each group are summarized in Table 1. Mean age of the DME and control patients was 59.7 ± 10.72 years and 61.3 ± 6.38 years, respectively. In the DME group, 62 patients (87%) had a history of high blood pressure and 11 (15%) had hyperlipidemia. In the control group, 7 patients (70%) had high blood pressure and 1 (10%) had hyperlipidemia. There were no significant differences between the groups with respect to age (P=0.646, Kruskal-Wallis test) or male-female distribution (P=0.789, Fisher’s exact test). Among patients with DME, there were no statistical differences among each DME group with respect to the duration of diabetes (P=0.079, Kruskal-Wallis test) and the stage (P=0.361, Fisher’s exact test).
Central macular thickness
The average CMT of each DME group at baseline was statistically different each other (P<0.001, Mann-Whitney test, Table 2). This may have been caused by varying morphologies of each DME type. Average CMT of all DME patients before IVB therapy was 453.99 ± 116.37 µm, which decreased to 336.07 ± 92.29 µm (P<0.001, Wilcoxon signed rank test) at 3 months after therapy. Each DME group also had a reduction in CMT at 3 months. The reduction ratio was calculated as (baseline CMT-3 month CMT)/baseline CMT. The following equation was used:
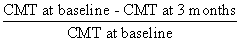
The reduction ratio was highest in the SDRT group (29.51%) followed by the CME group (26.59%), and then the combined group (24.42%). The reduction was the lowest in the SRD group (15.02%). Reductions in CMT were not statistical different between the SDRT, CME, and combined groups (P=0.498, Kruskal-Wallis test). However, the CMT reduction observed in the SRD group was significantly lower than in the combined group, which was the third highest (P=0.014, Mann-Whitney test) (Table 2 and Figure 2).
| DME groups | P-value* | |||||
|---|---|---|---|---|---|---|
| SDRT | CME | SRD | Combined | All | ||
| Baseline CMT (µm) | 402.63±69.08 | 511.22±104.50 | 392.40±53.63 | 534.00±158.37 | 453.99±116.37 | <0.001 |
| 3-month CMT (µm) | 278.41±55.11 | 367.06±68.09 | 334.93±63.07 | 399.56±130.06 | 336.07±92.29 | <0.001 |
| % reduction in CMT from baseline | 29.51±15.03 | 26.59±13.25 | 15.02±7.59 | 24.42±11.84 | 24.89±13.59 | 0.008 |
| Baseline logMAR VA | 0.70±0.12 | 0.69±0.68 | 0.71±0.17 | 0.72±0.16 | 0.70±0.13 | 0.915 |
| 3-month logMAR VA | 0.49±0.11 | 0.51±0.12 | 0.57±0.13 | 0.59±0.14 | 0.53±0.13 | 0.040 |
| Improvement in logMAR VA over baseline | -0.21±0.14 | -0.18±0.12 | -0.14±0.15 | -0.13±0.18 | -0.17±0.15 | 0.319 |
Table 2: Central macular thickness and visual acuity at baseline and 3 months after intravitreal bevacizumab.
Figure 2: (Left) Central macular thickness (CMT) reduction (%) and (Right) Improvement in the logarithm of the minimum angle of resolution (logMAR) visual acuity (VA) following intravitreal bevacizumab (IVB) in diabetic macular edema (DME) groups. Diffuse retinal thickening (SDRT), cystoids macular edema (CME), and combined DME patients had a greater reduction in CMT than serous retinal detachment (SRD) patients. There were no statistical differences among the SDRT, CME, or Combined groups (P=0.498, Kruskal-Wallis test). The percent reduction in the SRD group was significantly lower than that in the combined group (P=0.014, Mann-Whitney test). Improvements over preoperative values were statistically significant in all groups (P<0.001, Wilcoxon signed rank test). No statistically significant differences in logMAR VA improvement were found between the four groups (P=0.213, Kruskal-Wallis test). The horizontal bar indicates the mean percent reduction and logMAR VA improvement in the DME groups.
Visual acuity
The baseline logMAR BCVA in all DME groups was 0.70 ± 0.13. There was no difference among DME groups prior to IVB therapy (P=0.915, Kruskal-Wallis test). The logMAR BCVA after 3 months was 0.53 ± 0.13, which was significantly improved compared to baseline (P<0.001, Wilcoxon signed rank test). The BCVA measurements before and after IVB for each group are shown in Table 2. Improvements from BCVA for each group during the first 3 months of IVB therapy are also shown in Table 2 and Figure 2. Visual acuity changes were not statistically different among DME groups (P=0.213, Kruskal-Wallis test).
Aqueous humor cytokines and vascular endothelial growth factor
Interleukin-6: Mean aqueous levels of IL-6, 8, 10, 13, MCP-1, and VEGF are shown in Table 3. Overall, the levels of all cytokines in each DME group were significantly higher than in the control group (P<0.001, Mann-Whitney test). Moreover, there were significant differences among the DME groups (P<0.001, Kruskal-Wallis test).
| Control (n=10 ) | DME group | P-values*† | ||||||
|---|---|---|---|---|---|---|---|---|
| SDRT (n=27 ) | CME (n=18 ) | SRD (n=15) | Combined (n=16) | All (n=76) | DME vs. control* | Among 4 DME groups† | ||
| IL-6 | 2.08±1.67 (0.16-4.68) | 69.22±51.22 (10.45-231.37) | 114.31±31.42 (73.93-189.23) | 36.01±26.19 (6.36-89.32) | 98.09±34.66 (38.25-189.32) | 79.42±47.89 (6.36-231.37) | <0.001 | <0.001 |
| IL-8 | 6.14±2.45 (3.28-0.04) | 25.86±18.17 (8.34-84.13) | 26.34±9.99 (11.65-44.87) | 22.70±10.66 (10.91-40.69) | 20.87±11.34 (9.98-48.67) | 24.30±13.74 (8.34-84.13) | <0.001 | 0.531 |
| IL-10 | 0.43±0.47 (0.04-1.53) | 1.18±0.79 (0.09-2.69) | 1.48±1.01 (0.19-4.20) | 1.71±1.17 (0.43-5.56) | 1.50±0.75 (0.43-3.12) | 1.42±0.92 (0.09-5.56) | <0.001 | 0.556 |
| IL-13 | 0.78±0.31 (0.32-1.31) | 0.93±0.38 (0.32-2.36) | 0.94±0.23 (0.53-1.45) | 0.95±0.29 (0.48-1.65) | 1.05±0.23 (0.70-1.45) | 0.96±0.30 (0.32-2.36) | <0.001 | 0.447 |
| MCP-1 | 723.25±146.24 (531.74–892.33) | 1812.69±393.42 (1277.65-2495.45) | 2195.58±382.52 (1556.76-2796.34) | 1594.62±327.88 (1129.78-2137.33) | 1690.53±448.40 (1032.45-2455.34) | 1834.61±441.08 (1032.45-2796.34) | <0.001 | 0.001 |
| VEGF | 21.91±9.42 (10.98-36.55) | 54.71±15.96 (28.32-89.84) | 50.75±14.68 (23.43-75.34) | 31.97±9.82 (18.49-46.54) | 49.21±21.11 (24.50-98.12) | 48.13±17.72 (18.49-98.12) | <0.001 | <0.001 |
Table 3: Baseline cytokine levels in the aqueous humor in the diabetic macular edema and control group.
The sequence of highest to lowest IL-6 levels was CME, combined, SDRT, and SRD (Figure 3). The CME and combined groups each had statistically higher levels of IL-6 than the SDRT group (CME vs. SDRT P=0.001, combined vs. SDRT P=0.025, Mann-Whitney test), but levels in the CME and combined groups were not significantly different (P=0.237, Mann-Whitney test).
Figure 3: Aqueous levels of Interleukin (IL)-6, 8, 10, 13, Monocyte chemoattractant protein (MCP)-1, Vascular endothelial growth factor (VEGF) in all groups. All the levels of cytokines in each DME group were significantly higher than in the control group (P<0.001, Mann-Whitney test, respectively) (Top left) The level of IL-6 in the CME and combined groups were significantly higher than in the SDRT group (CME vs. SDRT, P=0.001; combined vs. SDRT, P=0.025, Mann-Whitney test). No statistically significant differences in IL-6 levels were found between the CME group and the combined group (P=0.237, Mann-Whitney test). IL-6 level in the SRD group was significantly lower than in the other DME groups (CME vs. SRD, P<0.001; combined vs. SRD, P<0.001; SDRT vs. SRD, P=0.036, Mann-Whitney test). (Top right) (Middle left) (Middle right) There were no statistically significant differences of the levels of IL-8, IL-10, IL-13 among the four DME groups (P=0.531, P=0.556, P=0.447, Kruskal-Wallis test, respectively). (Bottom left) The MCP-1 levels were significantly higher in the CME group than in the SDRT, SRD, and combined groups (CME vs. SDRT, P=0.004; CME vs. SRD, P<0.001; CME vs. combined, P=0.003, Mann-Whitney test). No statistically significant differences in MCP-1 levels were found among the SDRT, SRD, and combined groups (P=0.215, Kruskal-Wallis test). (Bottom right) The levels of VEGF were significantly higher in the SDRT, CME, and combined groups than in the SRD group (SDRT vs. SRD, P<0.001; CME vs. SRD, P<0.001; combined vs. SRD, P=0.006, Mann-Whitney test). No statistically significant differences in VEGF levels were found among the SDRT, CME, and combined groups (P=0.393, Kruskal-Wallis test).
The SDRT group had higher levels of IL-6 than the SRD group (P=0.036, Mann-Whitney test) and the SRD group had higher levels than the control group (P<0.001, Mann-Whitney test). Interestingly, the SRD group had significantly lower IL-6 levels than the other DME groups. Univariate and multivariate binomial logistic regression analysis showed that IL-6 levels were associated with the development of CME (P=0.005, odds ratio [OR]=1.027, 95% confidence interval [CI]=1.008-1.046), SRD (P=0.045, OR=1.018, 95% CI=1.006-1.030), and combined (P=0.031, OR=1.013, 95% CI=1.002-1.024), as shown in Tables 4-7.
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Odd ratio (95% CI) | P-value* | Odd ratio (95% CI) | P-value* | |
| IL-6 | 0.999 (0.990–1.008) | 0.882 | ||
| IL-8 | 1.026 (0.994–1.060) | 0.114 | ||
| IL-10 | 0.793 (0.468–1.343) | 0.388 | ||
| IL-13 | 0.835 (0.183–3.817) | 0.816 | ||
| MCP-1 | 1.001 (1.000–1.001) | 0.222 | ||
| VEGF | 1.043 (1.015–1.072) | 0.003* | ||
Table 4: Factors related to SDRT, as determined by age and sex adjusted logistic regression analysis.
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Odd ratio (95% CI) | P-value* | Odd ratio (95% CI) | P-value* | |
| IL-6 | 1.025 (1.011–1.039) | <0.001* | 1.027 (1.008–1.046) | 0.005* |
| IL-8 | 1.024 (0.990–1.060) | 0.173 | ||
| IL-10 | 1.263 (0.746–2.137) | 0.385 | ||
| IL-13 | 1.024 (0.186–5.647) | 0.979 | ||
| MCP-1 | 1.003 (1.001–1.004) | <0.001* | 1.003 (1.001–1.006) | 0.002* |
| VEGF | 1.020 (0.992–1.048) | 0.045* | ||
Table 5: Factors related to CME, as determined by age and sex adjusted logistic regression analysis.
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Odd ratio (95% CI) | P-value* | Odd ratio (95% CI) | P-value* | |
| IL-6 | 1.018 (1.006–1.030) | 0.045 | ||
| IL-8 | 1.003 (0.965–1.043) | 0.878 | ||
| IL-10 | 1.642 (0.936–2.881) | 0.084 | ||
| IL-13 | 1.164 (0.191–7.082 | 0.869 | ||
| MCP-1 | 1.000 (0.999–1.001) | 0.390 | ||
| VEGF | 0.942 (0.903–0.982) | 0.156 | ||
Table 6: Factors related to SRD, as determined by age and sex adjusted logistic regression analysis.
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Odd ratio (95% CI) | P-value* | Odd ratio (95% CI) | P-value* | |
| IL-6 | 1.013 (1.002–1.024) | 0.023* | 1.013(1.002–1.024) | 0.031* |
| IL-8 | 0.991 (0.952–1.033) | 0.679 | ||
| IL-10 | 1.287 (0.748–2.216) | 0.362 | ||
| IL-13 | 3.744 (0.666–21.039) | 0.134 | ||
| MCP-1 | 1.000 (0.999–1.001) | 0.904 | ||
| VEGF | 1.014 (0.986–1.043) | 0.033* | 1.012 (0.988–1.036) | 0.038* |
Table 7: Factors related to combined CME and SRD by age and sex adjusted logistic regression analysis.
Interleukin-8, 10, 13: Mean aqueous levels of IL-8, 10, 13 are shown in Table 3. The levels of IL-8, 10, 13 among the DME groups were not statistically different (P=0.531, P=0.556, P=0.447, Kruskal-Wallis test, respectively) (Table 3 and Figure 3). Also, univariate and multivariate binomial logistic regression analysis revealed that IL-8, 10, 13 levels were not statistically associated with the development of any DME.
Monocyte Chemoattractant Protein-1: Mean aqueous levels of MCP-1 are shown in Table 3. Each DME group had significantly different levels (P=0.001, Kruskal-Wallis test) (Table 3 and Figure 3). A post-hoc comparison revealed that the CME group had the highest MCP-1 levels of all DME groups (CME vs. SDRT P=0.004, CME vs. combined P=0.003, CME vs. SRD P<0.001, Mann-Whitney test). The MCP-1 levels were not significantly different between the SDRT, SRD, and combined groups (P=0.215, Kruskal-Wallis test). Additionally, univariate and multivariate binomial logistic regression analysis showed that MCP-1 was associated with the development of CME (P=0.002, OR=1.003, 95% CI=1.001-1.006, Table 5).
Vascular endothelial growth factor: Mean aqueous levels of VEGF are shown in Table 3. Each DME group had significantly different levels (P<0.001, Kruskal-Wallis test) (Table 3 and Figure 3). A post-hoc comparison revealed that the VEGF level in the SDRT, CME, and combined groups were significantly higher than that in the SRD group (SDRT vs. SRD P<0.001, CME vs. SRD P<0.001, combined vs. SRD P=0.006, Mann-Whitney test). There was no statistically significant difference in the VEGF level between the SDRT, CME, and combined groups (P=0.393, Kruskal-Wallis test). Univariate and multivariate binomial logistic regression analysis showed that VEGF was associated with the development of SDRT (P=0.003, OR=1.043, 95% CI=1.015-1.072) and combined (P=0.038, OR=1.012, 95% CI=0.988-1.036) groups (Tables 4,7).
Correlation analyses
>The relationships between the severity of diabetic macular edema on OCT and aqueous levels of cytokines were analyzed in each group, which was significant with the development of DME by univariate and multivariate binomial logistic regression analysis. Interestingly, in the CME and combined groups, the correlation coefficients corresponding to the relationships between CMT and levels of VEGF were statistically significant (CME group: r=0.346, P=0.045, combined group: r=0.559, P=0.024, Spearman’s correlation test) (Figure 4). None of the other cytokines were significantly correlated with CMT.
Figure 4: Scatterplots of the correlations between central macular thickness (CMT) and aqueous humor concentration of cytokines in each group that cytokines were related with development of each DME pattern. The aqueous concentrations of VEGF were correlated significantly with the degree of central macular thickness in the CME group (D) (r=0.346, P=0.045) and in the combined group (G) (r=0.559, P=0.024). The other cytokines were not correlated with the degree of CMT in OCT of SDRT, CME, SRD and combined pattern. VEGF in the SDRT (A) (r=-0.096, P=0.634), IL-6 in the CME (B) (r=0.164, P=0.515), MCP-1 in the CME (C) (r=0.247, P=0.288), IL-6 in the SRD (E) (r=-0.189, P=0.499), IL-6 in the combined group (F) (r=0.291, P=0.274).
Diabetic patients are in a low state of chronic inflammation that is associated with hyperglycemia. As the inflammatory level related to diabetes increases, chronic inflammation associated with cytokines would cause many complications eventually and DME can be one of the typical examples [12,13]. However, the precise role of these inflammatory factors, including cytokines and VEGF, related with DME is not fully understood. It is known that several inflammatory factors, including IL-6, IL-8, MCP-1, and VEGF, cause an increase in permeability of blood vessel, which leads to an increase in central macular thickness [14-18]. Our results are in agreement with previous studies, which have shown a relationship between DME and intraocular inflammatory cytokine levels [14-19], and which have recently reported that IVB and IVTA have different effects on different types of DME, classified according to OCT patterns [9,20]. Our findings demonstrate the cause of different results determined on OCT and the precise role of inflammatory cytokines in the pathogenesis of DME.
Sponge-like diffuse retinal thickening
It is generally accepted that SDRT formation begins with a persistent breakdown of the inner retinal blood-retinal-barrier, which results in diffuse fluid accumulation in the extracellular space of retinal tissue [21]. The decrease in blood-retinal-barrier likely occurs from a loss of tight junction anchor proteins in capillary endothelial cells. It is known that VEGF, is related to angiogenesis, vascular permeability, and macular edema severity as well [17,18]. It is also known that the VEGF level in both the vitreous and aqueous humor is associated with retinal vessel permeability and DME severity [16]. As a result, VEGF causes extracellular fluid accumulation from the intravascular compartment through intracellular tight junction disruption [17, 21-24]. This study demonstrated that the level of aqueous VEGF was particularly high in the SDRT group and only VEGF is known to be related to SDT development. Therefore, anti-VEGF therapy is particularly effective for SDRT, as also confirmed by other reports [9].
Cystoid macular edema
The pathogenesis of CME has not been clearly identified yet, but evidence suggests that VEGF is related to CME formation. Though not as effective as in SDRT, IVB therapy is effective in treating CME [9]. Our results are consistent because VEGF levels were elevated in the CME group and IVB therapy was effective, not as effective as in the SDRT group. Although, the relationship was not significantly difference in multivariate binomial logistic regression analysis, univariate binomial logistic regression analysis showed that VEGF is related with CME development and also the concentration of VEGF was significantly associated with the degree of central macular thickness on OCT (r=0.346, P=0.045, Spearman’s correlation test) (Figure 4). These significant correlations between the VEGF concentration and the macular thickness suggest that the elevated VEGF concentration were associated with the severity of diabetic macular edema.
Pro-inflammatory cytokines, including IL-6 and IL-8, were present in higher levels in the CME group than in other groups. Interluken-6 is very important in controlling the immune system and plays a major role in acute inflammatory reactions. It is also known that IL-6 is produced by a variety of cells (e.g., hair cells, monocytes, T-lymphocytes, B-lymphocytes, vascular endothelial cells) and is related to increased VEGF expression [15]. A previous study showed that IL-6 plays a role in neovascularization in proliferative diabetic retinopathy and in DME onset [14]. Funatsu et al. [15,16] found that aqueous IL-6 levels were directly correlated with increases in central retinal thickness and macular volume, as measured by OCT. We also found that increased IL-6 levels induce CME through multivariate binomial logistic regression analysis.
The CME group had the highest level of MCP-1 in the current study. Retinal endothelial cells produce MCP-1, a chemotactic cytokine that plays a major role in vessel wall monocyte replenishment following vascular injury [19]. Previous studies have shown that aqueous MCP-1 levels increase as diabetic retinopathy progresses [25] and that MCP-1 levels are elevated in eyes with DME [26]. In addition, there is a report demonstrating the relation of MCP-1 with DME [16,27]. Our study showed that MCP-1 is thought to play a role in CME development. MCP-1 related with vascular injury and various factors associated with permeability. Therefore, the increase in VEGF and other pro-inflammatory cytokines in the CME group suggest that IVB and IVTA would be effective in treating CME like the results of previous study [20].
Serous retinal detachment
Although the SRD pathogenic mechanism remains unclear, it is generally thought that SRD develops because of fluid movement from the edematous retina to the subretinal space or from outer blood-retinal-barrier breakdown at the RPE [28]. According to Kang et al. [29], SRD results from ineffective fluid and/or albumin removal by the RPE, even though a large volume of fluid moves through the permeable external limiting membrane (ELM). The results of several studies [28,30-32] suggest that the fluid pumping capabilities of the RPE are reduced during hypoxia and that RPE impairment plays an important role in SRD development. Furthermore, when the ELM is disrupted, fluid accumulates anterior to the ELM, inducing outer retinal swelling and eventually SRD [33]. In this study, IL-6 was related to the development of SRD. We believe that diabetes-induced RPE impairment and ELM disruption caused accumulation of inflammatory cells, damaged cells, and debris, which may have been exacerbated by elevated cytokine levels, including that of IL-6. Ultimately, these changes could have aggravated the inflammation reaction and disrupted the ELM and RPE.
Combined pattern
In the combined pattern, consisted of eyes with both CME and SRD, we might expect that there are characteristics shown on CME and SRD patterns. Consequently, CME developed from increased cytokine levels and from RPE and ELM function impairment, which corresponded to an increased vascular permeability. Our results confirmed that increased VEGF and IL-6 levels correlated with the development of combined pattern. Furthermore, the concentration of VEGF was significantly associated with the degree of central macular thickness on OCT (r=0.559, P=0.024, Spearman’s correlation test) (Figure 4). It showed that the concentration of VEGF had a strong correlation with macular edema severity.
Inflammatory and anti-inflammatory cytokines
Previous work has shown that IL-8 levels are higher in the vitreous of patient with proliferative diabetic retinopathy [33], which can result from hypoxia and promote neovascularization [34]. It has also been reported that vitreous levels of IL-8 were higher in patients with active proliferative diabetic retinopathy than inactive stage proliferative diabetic retinopathy [35]. Therefore, IL-8 is known to promote neovascularization and could play an important role in new vessel generation. Even though it remains controversial, many articles have reported a close relation between IL-8 and DME [36-38]. In the current study, levels of IL-8 were not statistically different among DME groups, but the level of IL-8 was statistically higher in DME patients than in the control group.
It is known that IL-10 is an anti-inflammatory cytokine that is produced by activated monocyte, macrophage, T lymphocyte, and B lymphocytes [39]. It has been shown to inhibit neovascularization resulting from hypoxia in animals [40], but the relationship between IL-10 and diabetic retinopathy is not clear [39]. Another study was shown that IL-10 levels are higher in eyes without diabetic retinopathy than in eyes with diabetic retinopathy [41], and another authors showed that vitreous IL-10 levels were increased in eyes with proliferative diabetic retinopathy [42]. Although we observed higher IL-10 levels in eyes with DME than in healthy control, but there was no difference among DME patterns in IL-10 levels. We assume that this compensatory increase in IL-10 was caused by hypoxic damage, which then triggered diabetes-induced inflammation. In other words, an increase in IL-10 means the late inflammation stages as inhibited process not with active stage.
Another anti-inflammatory cytokine is IL-13, which is produced by Th2 cells, mast cells, and natural killer cells. Along with IL-10, it is known to suppress expression of inflammatory cytokines, including IL-1, IL-6, IL-8, TNF-α, and IL-12, related to monocyte and macrophage action via increased expression of the MHC class II and CD23 genes [43]. A recent study showed increased vitreous IL-13 levels in patients with diabetic retinopathy [42]. Although no study to date has demonstrated a relationship with macular edema, it can be inferred that IL-13, like IL-10, is increased in the aqueous humor to compensate for increases in intraocular inflammatory cytokines (e.g., IL-6, IL-8, TNF-α, MCP-1) in patients with diabetic retinopathy [44]. Furthermore, it is known that IL-13 plays an anti-inflammatory role in the way that diabetic retinopathy is a chronic inflammatory disease. Therefore, it is possible that IL-13 may be effective in treating DME, as it is in reducing inflammation in eyes with uveitis [44]. In our study, both IL-10 and IL-13 were statistically higher in eyes with DME than in healthy controls. It is thought that IL-10 and IL-13 increases as a compensatory mechanism in DME, but no differences were found in IL-10 and IL-13 levels among DME groups. Further research is needed to determine the precise role of IL-10, IL-13 and the mechanism by which it acts in diabetic retinopathy.
In summary, the current study suggest that specific aqueous cytokines induce each DME pattern at early phase. They demonstrate that VEGF induces SDRT, that IL-6 and MCP-1 play a role in CME development, and that IL-6 is involved in SRD development. Furthermore, combined pattern is correlated with IL-6 and VEGF. These results may strongly suggest an association between these cytokines and the development of diabetic macular edema. Also, In CME and combined groups, the concentration of VEGF was correlated with the central macular thickness as surrogate of the degree of diabetic macular edema. These findings explain why IVB has different effects on the different DME. In the clinical setting, our results suggest that OCT imaging is important to choose the treatment of DME.
There are several imitations in our study. First, a relatively small number of patients were enrolled into the study. Despite of this, however, the results were statistically significant and clinically important. Second, we measured aqueous cytokine levels and not vitreous cytokine, which may be higher levels and better for the representative of the retina [44-46]. Third, cytokine levels were only measured before IVB therapy so the effect of treatments on cytokine levels was not examined. Fourth, many DME eyes show the combination of different OCT pattern and it was difficult to define the pattern in practice.
However, this study clearly demonstrates that various intraocular inflammatory cytokines and VEGF play in forming the different DME patterns at early phase. Especially, the analysis of inflammatory (IL-6, IL-8, MCP-1) and anti-inflammatory (IL-10 and IL-13) cytokines based on OCT pattern shows great meaning. More research is necessary on a larger group of patients to study both the natural DME process and the effect of various therapies on the different DME patterns.
This work was supported by the research grant of the Medical University of Hallym, Korea. The authors would like to thank Jenny Um, Bum Ho Shin for critical discussion. They also thank the Koma Biotechnology lab Unit, particularly Na Ra Shin for help with ELISA and Luminex multiplex bead assay on the aqueous humor specimen.