
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Review Article - (2021)Volume 11, Issue 3
Objective: Epithelial Ovarian Cancer (EOC) in early stage is difficult to diagnose. Serum indicators for stage I-II of epithelial ovarian cancer which confined to pelvic cavity were found through retrospective analysis, possible early detection methods might be found.
Methods: 165 patients were diagnosed as epithelial ovarian cancer at stage I-II from January 1st, 2015 to December 31st, 2019. Data was collected including age, pathological type, serum D-dimer (D-D), Neutrophil to Lymphocyte ratio (N/L), the platelet to Lymphocyte ratio (P/L), Cancer Antigen 125 (CA125), Human Epididymis Protein 4 (HE4) and diameter of the ovarian mass by ultrasound.
Results: D-D, CA125, HE4, ROMA, diameter, pathological type and age were significantly different in the different stage, age showed independent effect after logistical regression (P<0.05). D-D, CA125, HE4, ROMA, diameter, age and stage were significantly different in the different pathological type, and diameter showed significant independent influence on different pathological type after binary logistic regression (P<0.05).
Conclusion: CA125, HE4, ROMA, diameter of tumor, D-dimer and age were found significantly different between stage I and stage II, with age shows good effect on the diagnosis for stage II and diameter of tumor shows diagnostic value for non-serous ovarian cancer. Combined diagnosis may improve the diagnosis rate of early ovarian cancer.
Epithelial ovarian cancer; Diagnosis; Early-stage; Cancer antigen 125
With a survival rate below 45% of five-year and 15,000 death caused by it globally making ovarian cancer the 7th most common cancer and 8st most common cause of cancer death among women [1,2]. The five-year survival rate is 92% for women with stage I epithelial ovarian cancers while only around 30% for those with advanced-stage [3]. Therefore, early detection, early diagnosis and early treatment of ovarian cancer are very important, which can improve the prognosis of patients significantly. Though many patients were diagnosed with evaluated CA125 or HE4 value, still there are some patients who were in low level that they are diagnosed with ovarian cancer. Thus, the present study aimed to explore if there was a role for these parameters that were simple and accessible in the preoperative to find the early-stage epithelial ovarian cancer, especially in these who were in negative CA125 and HE4 value and make a judgement of the characteristic of the tumor.
In the recent years, there are more and more advanced equipment to detect pelvic mass, such as different kinds of Cancer Antigen (CA125, CEA, CA199, AFP), pelvic MRI [4,5]. When patients are suspicious for as a malignant tumor, more senior technology and consumption are truly needed. Completed blood count and ultrasonography for women are easy to get and relatively at low cost. Chronic inflammation has been associated with the carcinogenesis of different types of tumors, including ovarian cancer, and neutrophil, lymphocyte are common inflammation cells in blood cell. And neutrophilto- lymphocyte ratio and platelet-to-lymphocyte ratio are considered predictive factors for survival in ovarian cancer [6,7].
From January 1st in 2015 to December 31st in 2019, patients were hospitalized as epithelial ovarian cancer in The First Affiliated Hospital of Chongqing Medical University. After comprehensive surgical staging, 165 patients were diagnosed as epithelial ovarian cancer from stage Ia to stage IIa. Operations included hysterectomy with bilateral salpingo-oophorectomy, omentectomy, and lymphadenectomy, when mucinous cancer was confirmed, appendicectomy should be performed to keep comprehensive staging. Both abdominal surgery and laparoscopic surgery were acceptable. These who had adjuvant chemotherapy before surgery were excluded, non-epithelial and borderline tumors were not included. Patients’ data were collected through HIS 2.5 (hospital information system) including the general information (patient ID, age, menopause or not, diagnosis), complete blood counts (PLA-Platelet Count, NEU-Neutrophil Count, LYM-lymphocyte count), coagulation function (D-D, D-dimer), serum tumor maker (CA125-Cancer Antigen 125, HE4-Human Epididymis Protein 4), pelvic ultrasound measurement (D-diameter of tumor, tumor location), pathological data (degree and type of differentiation).
Samples
All the blood sample date was obtained before surgery and the nearest to surgery if there were more than one for analysis. The Complete Blood Count (CBC) was obtained through the automated hematology analyzer Sysmex XE-2100, X-800, According to our Receiver Operating Characteristic (ROC) curve analyses, the optimal cut off point for elevated PLT was considered if higher than 320, elevated NEU was considered if higher than 6.3, and elevated LYM was considered if higher than 3.2 (All the CBC count values were expressed in 109/L). In the meanwhile, we calculated the ratio of Neutrophils to lymphocytes (N/L) and ratio of platelets to lymphocytes (P/L). Sysmex CA1500, CA7000, CS5100 were used to detect the D-D value, which was presented in milligram per liter (mg/l) and if more than 0.55 mg/L was regard as elevated.
Serum CA125 and HE4 concentration were determined through the electrochemiluminescence technique in the Cobas e602 automatic analyzer. All CA125 values were expressed in units per milliliter (u/ml), and HE4 were expressed in picomole per liter (pmol/l). The levels of CA125 were considered elevated if higher than 35 u/ml. The HE4 value was thought elevated if higher than 70 pmol/l for premenopausal women while higher than 140 pmol/l was considered elevated for postmenopausal women. In the meanwhile, ROMA index was calculated through the Sysmex system according to menopause or not. Ovary cancer prediction value was low If the ROMA lower than 7.4% for premenopausal women and lower than 25.3% for postmenopausal women.
Diameter, location (in the left, right, or bilateral) and Resistance Index (RI) were recorded by pelvic ultrasound. The diameter of the ovarian mass was expressed in centimeter (cm). If more than one mass was detected on the ovarian, the diameter of the largest mass was chosen as the ovarian cancer’s diameter.
Pathological type, differentiation degree and clinical stage were collected as specific as possible. And the ovarian cancer staging was complied with surgical and pathological staging system of ovarian malignant tumor (FIGO, 2014) [8]. All the Pathological diagnosis report was given by the Pathology department, medical testing center, Chongqing Medical University.
Statistical analysis
The data were analyzed using Statistical Product and Service Solutions 25.0 (SPSS 25.0, IBM). Logistic regression was used to explore the risk factors of the early ovarian cancer, and predict the probability of ovarian cancer according to the risk factors, and p values<0.05 was considered as significant. Ovarian tumors were classified into stage I and stage II, serous and non-serous, with and without CA125 elevated group (CA125+, CA125-). The measurement data in accordance with normal distribution were expressed as mean ± standard deviation, and the comparison between the two groups was conducted by two independent sample t-test. The measurement data of nonnormal distribution were expressed as median (interquartile interval). Rank sum test was used to compare the two groups. The count data were expressed as percentage, and the comparison between groups was performed by chi square (χ2) test. Then we used factors that were significant different between two groups to do the logistic regression analysis.
General information and clinical features of EOC with stage I and stage II
Totally 165 cases were collected. All EOC patients diagnosed as stage I and stage II were located in pelvic, while patients with stage I candidate early stage, and with staged II means patients in advanced stage but limited in pelvic. More patients with stage II were found (103 vs. 62).
After normality test it can be found that D-D, CA125, HE4, ROMA, N/L, P/L, D-diameter and age do not comply to normal distribution (P<0.05), so rank sum test is used and LYM complies to normal distribution, then t test is used.
The rank sum test showed that D-D, CA125, HE4, ROMA, D-diameter and age are significantly different in the stage (P<0.05), while N/L, P/L show no significant difference in the stage (P>0.05).
Independent sample t test showed that there was no significant difference in LYM in the stage (P>0.05).
χ2 test showed that there were significant differences in different pathological type (P<0.05).
Among them, the proportion of patients classified as non-serous in stage I was relatively high (64.52%), and the proportion in stage II was relatively low. Chi square test showed that there was no significant difference between left and right in the stage (P>0.05) (Table 1).
| Stage I | Stage II | P | P* | |
|---|---|---|---|---|
| Number | 62 | 103 | 0.001 | |
| Age (yrs) | 49 (42.8-56) | 52 (46-58) | 0.041 | 0.012 |
| Menopause | 0.078 | |||
| No | 61.29% (38/62) | 47.06% (48/103) | ||
| Yes | 38.71% (24/62) | 53.92% (55/103) | ||
| Pathological type | 0.01 | |||
| Serous | 35.48% (22/62) | 55.34% (57/103) | 0.309 | |
| Mucinous | 33.87% (21/62) | 11.65% (12/103) | 0.422 | |
| Clear cell | 22.58% (14/62) | 21.36% (22/103) | 0.192 | |
| Endometrioid | 6.45% (4/62) | 8.74% (9/103) | 0.594 | |
| Others | 1.61% (1/62) | 2.91% (3/103) | 0.511 | |
| Location | 0.158 | |||
| Bio | 13.2% (8/62) | 22.8% (26/103) | ||
| Right | 39.2% (26/62) | 39.4% (39/103) | ||
| Left | 47.4% (28/62) | 37.8% (38/103) | ||
| Diameter (cm) | 12.55 (8.28-17.03) | 9.70 (7.50-12.87) | 0.005 | 0.044 |
| CA125 (u/ml) | 60.75 (22.23-178.90) | 211.60 (49.33-688.80) | 0 | 0.148 |
| HE4 (pmol/I) | 63.00 (45.50-90.50) | 113.00 (64.50-313.50) | 0 | 0.373 |
| ROMA (%) | 13.23 (7.55-31.39) | 55.09 (22.39-86.60) | 0 | 0.557 |
| D-D (mg/I) | 0.36 (0.18-1.07) | 1.03 (0.42-2.51) | 0 | 0.257 |
| N/L | 2.68 ± 1.77 | 3.39 ± 3.56 | 0.238 | |
| P/L | 169.94 ± 95.72 | 187.84 ± 127.61 | 0.299 |
Note: CA125: Cancer Antigen 125; D-D: D-dimer; HE4: Human Epididymis Protein 4; N/L: Neutrophil-to-Lymphocyte ratio; P/L: Platelet-to- Lymphocyte ratio; P*: P value after binary logistic regression
Table 1: General information and clinical features in stage I and stage II.
For the parameters with statistical differences including D-D, CA125, HE4, ROMA, D-diameter, age and type, they were tested by logistic regression as the risk factors. Age showed significant independent effect on the occurrence of stage (P<0.05). So, if patient detects with ovarian tumor, the aged is older than 50-year-old, serum CA125 is higher than 100 u/ml, HE4 is in positive level, D-D is high, more possibility is found to be advanced stage as malignant cancer. The ratio of the elder subgroup to the younger subgroup was 1.061 (Table 1).
According the two results of logistic regression analysis, we draw a Receiver Operating Characteristic (ROC) curve of the age to stage (Figure 1) and diameter to pathologic type (Figure 2). and Area Under the Curve (AUC) of a) is 0.803 and b) is 0.647. In the case of AUC higher than 0.5, the closer the AUC to 1 is, the better the diagnostic effect is. It proves there is a good effect on the diagnosis for stage II of age and diameter for non-serous ovarian cancer.
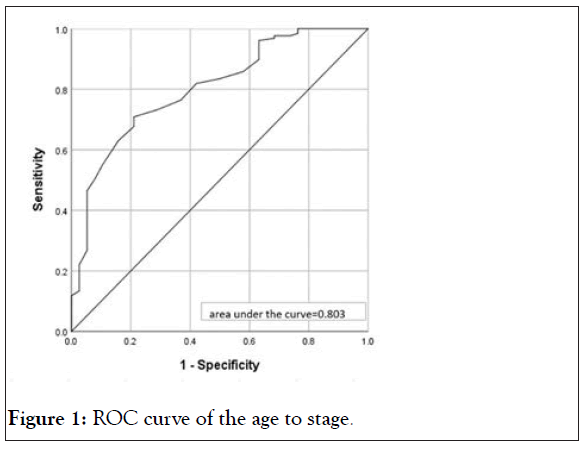
Figure 1: ROC curve of the age to stage.
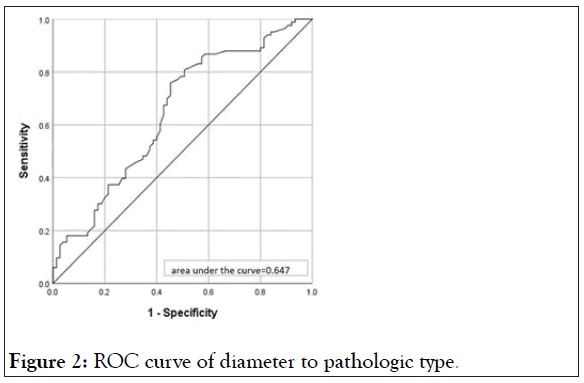
Figure 2: ROC curve of diameter to pathologic type.
Clinical features of EOC with CA125 level positive or not
Patients selected with CA125 value<35 was regarded as negative value. For ovarian epithelial malignant tumors confined to pelvic cavity, the number of CA125+ was more than twice the number of cases that in CA125-. In CA125 negative group, serum HE4, Roma, D-D was not significantly increased, indicating that the above serological indicators for supplement of CA125 for the diagnosis of early ovarian cancer are limited. The other clinical features of the stage showed that the location of ovarian cancer in CA125 negative group was more common on the right side, more than 50%, and the number of clear cell carcinoma in pathological type was significantly increased, reaching 36%, which was significantly higher than that in CA125 positive group. There was no significant difference in age and tumor diameter (Table 2).
| CA125- | CA125+ | P | |
|---|---|---|---|
| Number | 50 | 115 | 0 |
| Age (yrs) | 51 (44 -59) | 51 (45 -56) | 0.785 |
| Menopause | 0.502 | ||
| No | 48% (24/50) | 53.91% (62/115) | |
| Yes | 52% (26/50) | 46.09% (53/1115) | |
| Stage | 0.081 | ||
| I | 48% (24/50) | 33.04% (38/1115) | |
| II | 52% (26/50) | 66.96% (77/1115 | |
| Location | 0.029 | ||
| Bio | 12% (6/50) | 24.35% (28/1115) | |
| Right | 54% (27/50) | 33.04% (38/1115) | |
| Left | 34% (17/50) | 42.61% (49/1115) | |
| Pathological type | 0.04 | ||
| Serous | 34% (17/50) | 53.91% (62/115) | |
| Mucinous | 22% (11/50) | 19.13% (22/115) | |
| Clear cell | 36% (18/50) | 15.65% (18/1115) | |
| Endometrioid | 6% (3/50) | 8.7% (10/115) | |
| Others | 2% (1/50) | 2.61% (3/115) | |
| Diameter (cm) | 10.50 (7.30-13.00) | 10.00 (7.80-15.10) | 0.511 |
| D-D (mg/I) | 0.29 (0.15-0.65) | 1.05 (0.43-2.34) | 0 |
| HE4 (pmol/I) | 51.00 (40.00-70.00) | 121.00 (67.50-318.00) | 0 |
| ROMA (%) | 10.22 (6.70-17.30) | 58.67 (23.17-87.13) | 0.001 |
| N/L | 2.84 ± 3.56 | 3.25 ± 2.77 | 0.477 |
| P/L | 175.71 ± 155.48 | 183.46 ± 95.77 | 0.696 |
Note: CA125: Cancer Antigen 125; CA125-: CA125<35 (u/ml); D-D: D-dimer; HE4: Human Epididymis Protein 4; N/L: Neutrophil to Lymphocyte ratio; P/L: Platelet to Lymphocyte ratio
Table 2: Subgroup analysis for CA125 level positive or not.
Clinical features of EOC with different pathological type
According to the origin and differentiation of ovarian tumors, the different pathological types can be divided into serous and non-serous cancer. Thus, these parameters were analyzed by different pathological types (serous and non-serous). D-D, CA125, HE4, ROMA, location and diameter were significantly different in different pathological type after rank sum test (P<0.05), while N/L, P/L shows no significant difference in different type (P>0.05). According to x2 test, there were significant differences among different stage (P<0.05). In the location, the proportion of patients in the non-serous group was relatively high, and the proportion was 52.12% (86/165) (Table 3). There was no significant difference in the classification of menopause (P>0.05).
| Serous | Non-serous | P | |
|---|---|---|---|
| Number | 79 | 86 | 0.586 |
| Age (yrs) | 51 (45-58) | 51 (45-58) | 0.589 |
| Menopause | 1 | ||
| No | 51.9% (41/179) | 52.33% (45/86) | |
| Yes | 48.1% (38/179) | 53.49% (41/86) | |
| Stage | 0.016 | ||
| I | 27.85% (22/179) | 46.51% (40/86) | |
| II | 72.15% (57/179) | 53.49% (46/86) | |
| Location | 0 | ||
| Bio | 34.17% (27/179) | 8.14% (7/86) | |
| Right | 34.17% (27/179) | 44.19% (38/86) | |
| Left | 31.65% (25/179) | 47.67% (41/86) | |
| Diameter (cm) | 8.8 (6.90-13.10) | 11.40 (9.15-15.40) | 0.001 |
| D-D (mg/I) | 1.07 (0.44-2.31) | 0.43 (0.19-1.67) | 0.002 |
| CA125 (u/ml) | 283.6 (61.20-811.80) | 63.60 (22.60-183.00) | 0 |
| HE4 (pmol/1) | 113 (66.50-439.50) | 67.00 (47.50-137.00) | 0 |
| ROMA (%) | 50.54 (19.57-89.44) | 21.42 (9.82-66.88) | 0.001 |
| N/L | 2.85 ± 2.90 | 3.37 ± 3.14 | 0.154 |
| P/L | 170.13 ± 86.01 | 191.20 ± 138.77 | 0.479 |
Note: CA125: Cancer Antigen 125; CA125: CA125<35 (u/ml); D-D: D-dimer; HE4: Human Epididymis Protein 4; N/L: Neutrophil to Lymphocyte ratio; P/L: Platelet to Lymphocyte ratio
Table 3: Subgroup analysis for different pathologic type.
According to this five-year history, the number of advanced epithelial ovarian cancer was significantly more than that of early ovarian cancer. Between the stage I and stage II of epithelial ovarian cancer, significant difference was observed in D-dimer, CA125, HE4, ROMA index. Diameter and age while ratio of Neutrophils to Lymphocytes (N/L) and ratio of Platelets to Lymphocytes (P/L) didn’t showed Statistical difference. It indicated that EOC with early stage and advanced stage may have different serum indicators which included in CBC and coagulation.
CA125, HE4, ROMA index and D-dimer may be possible serum tumor markers in EOC early stage
Serum CA125 concentration test is the most widely studied biochemical method for screening ovarian cancer. About 50% of women with early ovarian cancer and more than 80% of women with advanced ovarian cancer have elevated serum CA125 value [9]. CA125 is the most common marker of epithelial ovarian cancer. The level of CA125 in these patients with stage II is higher than the normal range, and the average value is more than 200 u/ml, which is significantly higher than that in stage I, it indicated the sensitivity of CA125 in advanced epithelial ovarian cancer, which can be thought as good predict factor. But the research objects of serum CA125 test for ovarian cancer mainly focus on postmenopausal women, because the variation of CA125 with menstrual cycle [10,11] and the prevalence of benign gynecological diseases like endometriosis [12], pelvic inflammatory disease [13,14] in premenopausal women will lead to a significantly higher proba bility of false-positive results. There is evidence suggests that for postmenopausal women at general risk, screening only by annual CA125 measurement is not sufficiently specific, more specific indicators need to be combined.
HE4 and ROMA index ere possible parameters for ovarian cancer early diagnosis [15,16]. In this study, HE4 level reached to 113.00 pmol/l in stage II, because the above results denied the impact of menopause on early ovarian cancer, so high level of HE4 and ROMA indicating that their values largely which reflecting the malignant behavior of tumor. There were 115 of 165 patients (69.7%) showing elevated CA125 levels in this study, 63.2% of which in staging I represented positive value, while positive rate was significantly increased into 76.9% when combined CA125 with HE4, as a good testimony that combined diagnosis can improve diagnostic accuracy.
D-dimer is a major degradation product of polymerized fibrin after blood coagulation and plasmin processing. In this study, Ddimer in patients with stage II was significantly higher than that in patients with stage I, indicating that hyper-coagulation in patients with malignant tumors can indicate the degree of malignancy of tumors. Elevated plasma D-dimer levels usually indicate active fibrinolysis and are observed in several disorders, including arterial and venous thromboembolic disease, malignancy, and renal and liver disease [17,18]. Serum D-dimer has been reported as a useful diagnosis and prognosis parameter for several malignancies including ovarian cancer [19-21]. In addition, elevated platelet counts and thrombocytosis have been shown to actively affect tumor growth and metastasis in ovarian cancer. In this early ovarian cancer, D-dimer did show significant difference between stage I and stage II, which indicates a worse prognosis. Learning coagulation related examination results before comprehensive staging operation can provide a better judgment of the disease.
Many much studies show that the neutrophil-to-lymphocyte ratio has been recognized as a potent prognosticator for PFS and OS in ovarian cancer [22-24]. In addition, neutrophil-to-lymphocyte ratio has been suggested to predict the immune status of women in ovarian cancer [25]. However, in this study, neutrophil-tolymphocyte ratio and platelet-to-lymphocyte ratio didn’t presented significant difference whether in different stage or in different pathological type. Most of them were focusing on the predictive value of P/L and N/L in distinguishing benign masses and malignant tumors [26-27], There was no significant difference in the ratio of N/L and P/L: Blood cell count in the patients with stage I/II, suggesting that the related indicators of malignant tumor in blood cells are of limited value in the early diagnosis of epithelial ovarian cancer.
Different pathological types, different ages and different sites of ovarian tumors may be clinical indications to consider the benign and malignant ovarian, but it has no value for early diagnosis of EOC
According to the analysis of histopathology, immunohistochemistry and molecular genetics, there are five subtypes of epithelial ovarian cancer, including High Grade Serous Carcinoma (HGSC), Low Grade Serous Carcinoma (LGSC), endometrial carcinoma, clear cell carcinoma and mucinous cancer. HGSC and LGSC present different molecular pathogenesis and are two types of tumors with essential difference. However, it is believed that both tumors may originate from the precursor lesions of fallopian tube [28]. They were classified as serious cancer, and the rest were classified as no non-serous cancer. According to our study, non-serous cancer presented great diameter, this is because clear cell carcinoma is usually a large mass, and mucinous carcinoma can vary from 8 to 20 cm in size, but can also be larger [29].
In this study serous carcinoma was more than other types in stage I and II, which was more significant in stage II, reaching 55.34%. The second type is mucinous carcinoma. The proportion of patients in stage I was significantly higher than that in stage II. While HGSC is the most common type of ovarian cancer, accounting for 70%-80% of all ovarian malignant tumors, but the tumor volume is often not in big size [30]. Therefore, when the ovarian mass is found giant, it is particularly important to do a good job in practice of tumor free principle.
For CA125 negative patients, we should be alert to the occurrence of ovarian clear cell carcinoma. Therefore, for highrisk groups, such as recurrent ovarian endometriosis cyst, deep invasive endometriosis patients, it is necessary to further prevent the occurrence of Endometriosis Associated Ovarian Cancer (EAOC).
Age revealed independent influence on the ovarian stage. The average age of patients with early ovarian cancer was lower than that of patients in stage II. The incidence rate of epithelial ovarian cancer increased with age. But there was no significant difference in menopause. An analysis of data from the "nurse health study" found that the risk of epithelial ovarian cancer increased by about 2% for women under 50 years old and 11% for women aged 50 and above [3,17]. The median age at diagnosis of ovarian cancer was 63 years [1]. Thus, referring with our study-age can be an independent influence factor, when CA125 elevated is observed in an elder woman with pelvic mass, there is reason to believe she is suffering an ovarian cancer at least in early stage. Patients younger than 50 years old and with tumor diameter larger than 10 cm are more likely to be early epithelial ovarian cancer than advanced ovarian cancer.
Tumor location: Ovarian cancer took placed in left or right side, unilateral or bilateral. There was no significant difference between stage I and stage II.
Tumor diameter: The diameter of ovarian cancer in stage I is larger than that in stage II, indicating that the invasive ability of ovarian cancer tissue in advanced patients was better than that of ovarian tissue growth alone. At the same time, we found that if epithelial ovarian cancer patients have normal level of CA125, more incidence was taken placed in the right ovarian, which is statistically different.
Limitations
Whether these clinical phenomena are related to the origin of ovarian blood vessels in different parts, further research was needed. The diagnosis rate may be improved after combination of the above factors of early ovarian cancer, and there will be more references for clinical diagnosis and treatment.
In this retrospective analysis, CA125, HE4, ROMA, diameter of tumor, D-dimer and age were found significantly different between in early stage and in advanced stage which located in pelvic. CA125 is still the most specific tumor marker for early ovarian cancer and advanced stage II epithelial ovarian cancer. The serum levels of HE4, Roma and D-D may assist with CA125 level in early diagnosis if CA125 in normal level. The diagnostic value of N/L and P/L for ovarian cancer was not found. What’s more, age shows good independent effect on the diagnosis for stage II and diameter of tumor shows independent diagnostic value for non-serous ovarian cancer.
Author contributions
Qiang Yi: Data collecting and analyzing, manuscript writing; Yu Ran: Data collecting and analyzing; Cong Li: Project development, manuscript revise. All authors read and approved the final manuscript.
Funding support
No
Conflict of interest
The authors declare that they have no conflict of interest.
Ethic approval
All patients were consent to participate in the study like this: “I have read this informed consent form, and my doctor has explained the purpose, content, risks and benefits of this clinical trial to me in detail, and answered all the questions I asked. I have already understood this clinical study, and I am willing to participate in this study”. The study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (2020-636).
Citation: Yi Q, Ran Y, Li C (2021) Diagnostic Value of Serum Tumor Markers for Epithelial Ovarian Cancer Stage I-II: A Retrospective Analysis. J Clin Trials. 11:462.
Received: 23-Apr-2021 Accepted: 07-May-2021 Published: 14-May-2021 , DOI: 10.35248/2167-0870.21.11.462
Copyright: © 2021 Yi Q, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.