
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Review Article - (2020)Volume 10, Issue 6
Cell And Gene Therapies (CAGT) have become a market force across a continuum of therapeutic indications. The CAGT market value is expected to reach nearly $7 billion USD by 2027 with a cost-adjusted growth rate of nearly 20% according to leading market research indices. Given their complexity, scope and breadth, it is essential to effectively educate and train resources to perform the required CAGT clinical trial operational delivery functions, as the quality and integrity of this form of therapy directly impacts patient safety. Described herein are empirical data, experiences and best practices for establishing a CAGT training curriculum to help achieve clinical trial operational competencies, protocol compliance and better patient outcomes.
Cell and gene therapies; Cell; Clinical Trials
Why educating and training clinical delivery teams in CAGT is important
Published research has shown that up to 70% of reported adverse events across clinical trials are preventable, with over 60% of those related to technical errors (44%) and inappropriate diagnosis (17%) [1-5]. Given their complexity, CAGT trials can pose even greater risks, as top inspection findings show that these studies often fail to follow investigational plan (51%) and have inadequate and inaccurate records (33%), specifically in terms of chains of custody and identity [6]. These analyses suggest that clinical delivery teams could benefit from further knowledge. As such, CAGT operational education should not be isolated to delivery of a specific protocol, but rather is a dynamic lifecycle training platform that evolves in real-time with safety updates, new risk profiles and scientific advances, empowering process improvements throughout the study lifecycle.
Project management discipline states that for consistent success ‘one must apply the requisite knowledge, skills, tools, and techniques to project activities in order to meet or exceed stakeholder needs and expectations, while balancing their competing demands’ [7]. This is particularly true in CAGT clinical development in support of asset-specific education and patient-facing operations training. Not surprisingly, clinical development sponsors prefer operational teams with advanced training and knowledge as they believe a comprehensive understanding of CAGT is the best predictor of operational success.
Creating self-learning tools that address the continuum of needs across roles within an organization can facilitate growth of qualified teams to manage CAGT clinical trials. A comprehensive solution can establish a sound foundation on which to build CAGT protocol-specific knowledge, while building expertise within roles that have greater responsibility and oversight across functions. Development of basic and advanced training should be an engaging mix of standardized information, open-access primary research, and self-learner practicums that apply knowledge and test understanding. Based on experience, some key takeaways about training CAGT clinical trial teams are noted below.
Regulatory and site start-up
Being able to effectively strategize and coordinate CAGT trial submissions to regulatory bodies is essential to minimizing unnecessary delays to study initiation at sites and requires detailed knowledge of GMO and non-GMO requirements. Training operational delivery teams on country-specific requirements, as well as how to identify opportunities to accelerate, has been shown to shorten the timeline for each step to approval. These time savings can collectively open trials sooner than benchmarks, even when compared to conventional therapies and despite additional committee reviews at sites. Delivery teams should become proficient in their working knowledge per country, and translate lessons learned and into best practices.
CAGT logistics
Whether autologous or allogeneic cells, viral vectors or bacterial transposons or some combination of these, CAGT logistics is a set of critical path tasks with many responsibilities across various stakeholders due to their labile nature and the medical urgency to treat patients. With health authority-mandated audit trails for chains of identity and custody, operational team members must not only be trained in the key concepts and requirements of CAGT logistics but must also understand how to identify and adjust to risks in real-time. Some of this knowledge may be best gained through practical experience, but fundamentally preparing resources to (1) understand logistics workflows and risks, (2) determine thresholds for compliance, and (3) ensure patient safety are core functions that must be studied, practiced and understood before they are actually applied.
Patient treatment and near-term follow-up
With receipt of the CAGT investigational product (IP), clinical site workflows to screen and prepare the patient for treatment begin in earnest. Successful receipt of the IP requires team members to know and understand (1) what patient risks to this form of therapy may now be present, (2) how to properly and compliantly unpackage and prepare the IP for patient treatment, (3) requirements for correct and compliant dose administration, and (4) what required documentation for administration and follow- up should be completed and when. In the event of unsuccessful receipt of IP at the site (temperature excursion, damaged packaging, uncharacteristic appearance), immediate, pre-planned actions must be in place. Experience has shown that site team members spend the most time training on these aspects of protocol conduct, as it is a critical time for the patient, who may not be able to encounter any delays in dosing.
Data currency and administrative compliance
CAGT casebooks can be up to 50% larger than those for conventional therapies and with up to 80% of patient data to be entered within the first weeks around dosing. Given this short timeline, the ability of teams to stay on top of the data from the outset of treatment initiation is more than just a challenge, and requires focused training, preparation and knowledge sharing in order to meet data entry and administrative compliance requirements. Working closely with study monitors and data managers, delivery teams should focus their training on protocol feasibility, potential data risks, study timelines and safety reporting requirements. Leading CAGT sponsors have stated that this training is the most important in terms of risk assessment and gap analysis for their entire clinical program.
Long-term follow-up and patient retention
Health authorities require varying periods of CAGT long-term follow-up. Operational teams should therefore be prepared to support patients long after dosing (up to 15 years), as these data are central to understanding the long-term effects of therapy on a patient’s well-being and quality of life, as well as a sponsor’s ability to successfully file for marketing authorization. Understanding requirements for duration of follow-up, the types of assessments that must be performed, and the data that should be collected are critical to patient safety and clinical development of CAGT.
For each organization and its stakeholders, it is important to address three questions: (1) What do resources need to learn about CAGT? (2) How will their understanding and knowledge of CAGT be assessed? and (3) What measures will be used to determine their application of that knowledge in terms of performance? With those questions addressed, a blueprint for a specific curriculum can be developed.
Frequency and setting
Given the rapidly evolving science, CAGT teams that meet frequently, i.e. once a week or more, are typically the most capable performers, because practice and revision of their understanding, planning and actions are continuously cultivated and revised. The group setting is an essential forum, so that knowledge can be crowd sourced and shared in real-time, offering the greatest opportunities to improve knowledge and increase performance.
Standardized training platform
While effective training on CAGT can take many forms, the most common approach is a standardized training platform that is self-contained and comprehensive. Individual, detailed training modules with information that the learner can reason and use to make logical connections helps to ensure their understanding of CAGT fundamentals (Table 1). Additional modules may choose to introduce more advanced concepts regarding patient safety decision algorithms or clinical pathway practicums that further develop their level of understanding and application and should culminate with a standardized knowledge assessment to formally document the learner’s progress and potential.
| Suggested training modules |
|---|
| Introduction to cell and gene therapy: Basic concepts |
| Cell and gene therapy operations: Best practices and lessons learned |
| Clinical monitoring of cell and gene therapy clinical trials |
| Cell and gene therapy advanced operational concepts and trailing challenges |
| Cell and gene therapy operations: Literature reviews and case studies |
| CAGT knowledge assessment |
Table 1: Suggested Training Modules for establishing a CAGT training curriculum within your team or organization.
Portable information
Through the use of detailed information on demand, CAGT team members can readily review and action both basic and advanced concepts. Examples of these include indication- and therapeutic platform-specific ‘pocket references’ (Figure 1), patient safety algorithms, and protocol process maps (Figure 2). With each of these supports, CAGT team members can be empowered in the moment of need or indecision with standardized information, technical detail and practical guidance.

Figure 1: A portable gene therapy primer for operational resources to reference in terms of key concepts and practical guidance.
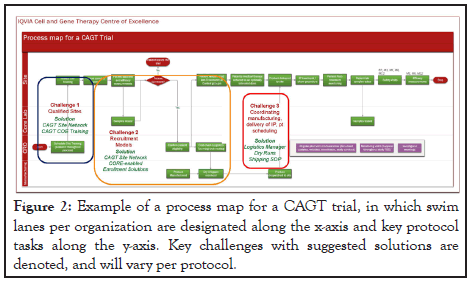
Figure 2: Example of a process map for a CAGT trial, in which swim lanes per organization are designated along the x-axis and key protocol tasks along the y-axis. Key challenges with suggested solutions are denoted, and will vary per protocol.
Practicums and knowledge assessments
Written practicums and standardized knowledge assessments may be the most useful evaluation methods available for determining a team members capability to perform workflows in support of CAGT [8]. While education and training should be lifecycle commitments in CAGT clinical development, it is still a best practice to measure and assess team member knowledge and capability before they actually begin assigned responsibilities.
Assessing performance and measuring improvement in a CAGT training curriculum should incorporate an understanding of the individual learner, team and greater organization so as to identify and define gaps and how to best address [9]. Frameworks measuring integration of CAGT knowledge and application for specific CAGT workflows will create a better understanding of curriculum benefits or deficiencies. Progress and improvement should encompass a variety of key performance indices (KPI), and as gaps are identified, these should not be evaluated in isolation, but can identify other potential causes, relationships and risks.
Addressing gaps: knowledge vs. performance
Once a training curriculum is implemented, it is important to differentiate gaps in knowledge (concepts, interrelationships, risks) from those in performance (areas of process, abilities and competence), and which impediment has the largest gaps. Gap analysis is intended to highlight the amount by which the need exceeds the resources that exist and what gaps may need to be filled to be successful [10]. Based on this, the next step to strengthen existing curriculum deficiencies is to determine whether a goal is achievable given the resources available.
Creating accessible solutions
Evaluating and adjusting team performance in real-time is a necessity, such that making changes to the CAGT training curriculum is often required. In doing so, updates in real-time benefit from rapid, simple messaging, and accessible curriculum proctors that can support ad hoc questions and understanding, while translating difficult concepts with the requisite detail and depth necessary to inform performance.
Creating a sense of a learning community
To effectively build a continuous learning community, it is important to promote professional development within teams around specific needs in CAGT operations. In doing so, professional development becomes a regular activity where the most effective learning forums embrace collaboration built on trust.
Metrics-driven performance evaluation
While KPIs often define operational performance, a key measure of performance that should be included is patients’ perceptions of care. These perceptions are an effective way to further engage both patients and investigators about their CAGT experiences and preferences, while also obtaining a deeper understanding than simple measures of patient satisfaction. CAGT training curriculums should evolve over time in response to metrics-driven performance, including the patient experience as part of that overall evaluation.
Curriculum commitments: educate, evaluate, improve, repeat
When implementing a culture of continuous learning in CAGT operational delivery, there are several steps that tend to be indispensable. First, gain a commitment for CAGT knowledge development. This is central to what makes this work, and everyone will need to be moving toward the same goal. Second, enable CAGT teams to meet routinely to talk about how to implement best practices or improve current processes. Continuous improvement requires regular practice. Third, tie delivery back to job performance, as true continuous improvement is metricsdriven. Finally, institute repetitive practices of learning, application and real-time evaluation.
CAGT clinical development is a complex operation, requiring continuous learning, performance evaluation and revision. It is essential to efficiently educate and train CAGT team members to routinely perform CAGT operational functions effectively, as the quality and integrity of this form of therapy directly impacts patient safety and clinical outcomes.
Citation: Learn CA, Stewart TD, Shah MR (2020) Developing an Effective Training Curriculum for Cell and Gene Therapy Operational Teams. J Clin Trials 10:432.
Received: 09-Sep-2020 Accepted: 21-Sep-2020 Published: 28-Sep-2020 , DOI: 10.35248/2167-0870.20.10.432
Copyright: © 2020 Learn CA, et al. This is an open−access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.