Mycobacterial Diseases
Open Access
ISSN: 2161-1068
ISSN: 2161-1068
Research Article - (2019)Volume 9, Issue 2
Out of 442 cases of rifampicin resistant MTB strains 410 cases showed Isoniazid resistance. Out of which exclusive involvement with inhA was seen in 32 cases. 19 cases showed involvement of both inhA as well as katG resistance 359 cases accounting to 87.5% showed exclusive involvement of katG thereby conferring high degree of resistance. The higher percentage of katG induced Isoniazid resistance among the Rifampicin resistant strains depicts the spread of higher degree of Isoniazid resistance. The question remains how effective would be the
Isoniazid prophylaxis in pediatric age group in this scenario. The analysis has shown that only around 14.93% cases reflected exclusive mutations at around 15 and 16 regions of the inhA gene and MUT3 accounted to a percentage of 60.3% among the inhA dependant isoniazid resistance.
INH resistance determining region mutation; katG inhA gene; TB Labs; Intermediate reference laboratory Kolkata; State TB Demonstration and Training Centre (STDC); Mycobacterium Tuberculosis Complex (MTBC); Retreatment preventive treatment ; INH prophylaxis; Contact cases
The preventive treatment of Tuberculosis is practiced worldwide in order to execute effective control on its spread. The need for a stream lined definite structured guideline is realized all over the world. Although there are several guidelines, proper rationale to the use of antibiotics as an effective prophylaxis is yet an area to be discussed. The practice of using an anti-tubercular drug without seeing resistance pattern and its rationality as a prophylaxis is at question.
Involvement of inhA and katG in conferring Isoniazid resistance among the Rifampicin resistant as well as sensitive cases were observed and compared to find out any correlation between resistance of Isoniazid and Rifampicin.
Moreover, treatment of drug resistant tuberculosis with the second line of anti-tubercular drugs has been a difficult affair. RNTCP (Revised national tuberculosis control program) advocates Isoniazid prophylaxis to the pediatric population who are at an augmented risk of getting the infection from close contacts, without knowing the resistant pattern of the strain of contacts. The study projects variation in the band patterns in inhA and katG among Rifampicin resistant adult and pediatric cases. The genetic patterns of isoniazid resistance and their different mutations among isolates of the cases obtained from the adult and pediatric age group of Rifampicin resistance are taken under the study. The point and periodic prevalence is worked out and considered in terms of evaluating the prophylaxis among the probable contacts of the source cases.
The guideline of managing of contacts of MDR TB and XDR TB patients has little scientific evidence to support and there is lack of national guidelines owing to the discrepancies as is strongly felt [1].
Pediatric contact groups are likely to have latent Tb infection that might follow an active TB disease development. Contacts of MDR TB are considered as LTBI cases. These cases are subjected to preventive therapy [2]. Most of the public health program follows the WHO guideline advocating the use Isoniazid as a prophylaxis irrespective of the resistant status of the source strain. Various national programs advocated administering of a conventional drug as a preventive therapy without actually knowing the susceptibility pattern of the infecting strain of the Source case [3].
The selection of a preventive regimen with single or multiple drugs ideally to be based on considering the availability and the bactericidal activity of the primarily infecting strain. Drug resistant TB among the new and retreatment cases was about 5% in accordance to the surveillance done. The overall proportion of multidrug resistant tuberculosis (MDR-TB), defined as TB resistant to at least Isoniazid (INH) and Rifampicin (RMP), with or without resistance to other firstline drugs, was 5.3%, ranging from 0% to 35% of reported TB cases. The percentage among the retreatment cases showed a steady rise over the years. The retreatment cases contributed about 20% of MDR TB among the high TB burden nation [4].
In 2009, only 30,000 (7%) of the 440,000 estimated MDR-TB cases globally were notified, and of them only 11,000 (3%) were put on treatment known to be consistent with international guidelines [5]. A systematic analysis of the nature of the resistance conferred towards Isoniazid may contribute in understanding the common as well as rare genetic alterations whereas corresponding Wild Type band is also positive MUT1 Pos along with WT 1 Pos contributed in 2% of the cases (with CI ± 2.52 at 95% Confidence limit) [6].
inhA codes for the Enoyl-ACP reductase of the type II fatty acid synthase (FAS-II) system. The enzyme helps in the biosynthesis of mycolic acids. Predominant component of mycobacterial cell walls [7]. Enoyl ACP Reductase Catalyzes the NADH-dependent reduction of the double bond of 2-trans-enoyl-[acyl-carrier protein], a fatty acid elongation step cycle of the FAS-II pathway. It is seen the exclusive point Mutation of the 15th base pair promoter sequence of inhA that converts Cysteine residue to threonine contributes the most among the inhA conferred resistance towards Isoniazid. katG codes for a virulence factor, and strains of M. tuberculosis without katG are heavily attenuated in animal models katG codes for peroxides which are required for protection against oxygen free radicals within the macrophage. Mutations have been described previously for the promoter region of katG .
Isoniazid resistances are classified as high and low level resistance based on the minimum inhibitory concentrations. It has been found that the high and low level resistance towards isoniazid is associated with the katG and inhA genes respectively. Treatment protocols are availed in accordance. The Isoniazid resistance is determined in all tubercular cases adult and pediatric irrespective of Rifampicin resistance status, it is found that 92.7% are Isoniazid resistant by katG among Rifampicin resistant cases. Whereas 17.8% of Isoniazid resistance by katG are even among Rifampicin sensitive cases. This relates to a community threat even more after implication of Isoniazid prophylaxis as a part of national program supported by WHO and a challenging threat of rising trend of Isoniazid resistance is apprehended.
Tuberculosis still remains the greatest killer disease of all times. The drug resistance has worsened the situation increasing the resistance towards the first line of drugs. As evident the scenario at district in India for Drug resistant TB among the new and retreatment cases was about 5% in accordance to the surveillance done in the year 2000 and 2001.
In 1994 in 35 countries the first WHO-IUATLD anti tuberculosis drug resistance surveillance was carried out. The study showed primary resistance and acquired multi drug resistance as 1.4% and 13% respectively. An estimate of 4, 89,139 cases of MDR-TB emerged in 2006 [8,9].
In July 2008, the US CDC during the MDR TB outbreak in Micronesia it was found out of the 232 contacts to 15 developed the Tuberculosis. On evaluation it was found that 66.7% of these patients carried the resistant strains. Preventive regimen with a resistant drug thus raises a fundamental question over the control of the disease [2].
The percentage among the retreatment cases showed a steady rise over the years. The retreatment cases contributed about 15 to 20% among the high Tb burden nations [10]. About 3% of the MDR cases. A systematic analysis of the nature of the resistance conferred may contribute in understanding the epidemiology of transmission of strains among the population. The study involves a probe into the most common patterns genetic mutations in inhA that has led to the Isoniazid resistance among the retreatment cases.
The majority among the inhA dependant Isoniazid mutations were seen to be occurring at the 15th codon of the inhA promoter region. The lower percentage of inhA dependant mutations with respect to the evaluated studies may prompt in a pear pressure for prolonged retreatment regimen might have converting several of low dose drug resistant strains to high dose drug resistant strains.
Moreover in high burden country with a high prevalence of the resistant strain the situation aggravates by using a resistant drugs for a prophylaxis. If the spread of the primary infection from the source case is by a strain resistant to, using that drug may be a futile exercise. Most of the countries have been practicing Isoniazid prophylaxis for children, contact and Latent tubercular infective cases.
The study explores the percentage prevalence of the Isoniazid resistance among the Rifampicin resistant source cases whose contacts are empirically subjected to Isoniazid.
The study was undertaken at the Intermediate reference laboratory under State TB Demonstration cum training Centre Kolkata, India under Revised National Tuberculosis control program, during April 2012 to December 2016. The study was done among the 442 Rifampicin resistant isolates recovered from retreatment cases in the year 2012 as well as 627 from newly diagnosed Rifampicin sensitive cases from a period of 2013-2016. The study involves a probe into the point and periodic prevalence of Isoniazid among the Rifampicin resistant as well as sensitive cases, the most common and rare patterns of genetic mutation that has led to the isoniazid resistance due to inhA as well as katG among the Rifampicin resistant and sensitive cases. The common and rare codons along with their change in amino acid sequences involved in conferring the resistance were looked for.
Materials and methods
The detection of the patterns genetic mutation is done by the Genotype MTBDR plus V2 kits (Hains Life Sciences) based on DNA strip technology (Line Probe Assay).
Reversed hybridization of the oligonucleotide probes to the designated gene segments of the rpoB gene was done for 442 isolates by line probe assay was done using the MTBDR Genotype Version 2. Reproducibility of LPA results was very high with 98.1% concordance of results between the two laboratories [8].
Smear positive samples were subjected to the Line probe assay directly after subsequent extraction of the genome and multiplex PCR of the specific gene segments using biotinylated primers [11]. The utility studies showed testing for Isoniazid by line probe Assay accorded to a sensitivity of 92.7% and specificity of 98.33% [12].
The multiplex PCR cycle involved 15 minutes at 95°C (1 cycle) for denaturation, 30 seconds 95°C and 2 minutes 65°C (20 cycles) for anealation, 25 seconds 95°C, 40 seconds 50°C, 40 seconds 70°C (30 cycles) for amplification and 8 min 70°C (1 Cycle) for extension (Tables 1 and 2) [8]. The targeted genetic segment was the inhA promoter region. Codon 8, 15 and 16 was looked for.
| Failing wild type band | Analyzed nucleic acid position | Developing mutation band | Mutation |
|---|---|---|---|
| inhA WT1 | -15 | inhA MUT1 | C-15T |
| -16 | inhA MUT2 | A-16G | |
| inhA WT2 | -8 | inhA MUT3A | T-8C |
| inhA MUT3B | T-8A |
Table 1: Mutation in the inhA promoter region and the corresponding wild type and mutation bands [8].
| Failing wild type band | Codon analyzed | Developing mutation band | Mutation |
|---|---|---|---|
| katG WT | 315 | katG MUT1 | S315T1 |
| katG MUT2 | S315T2 |
Table 2: Mutation in the katG gene and the corresponding wild type and mutation bands.
Among majority of cases considering all possible combinations it was found that combination exhibiting the Presence of MUT1 and absence of WT1 contributed to the most of the inhA dependant low level resistance conferred, (about 67.3%)involving the codon 15 and 16 of the promoter region of inhA gene.
Total of 442 Rifampicin resistant cases and 627 Rifampicin sensitive were studied out of which 52 M. tuberculosis complex strains showed resistance towards Isoniazid due to the involvement of inhA exclusively for Rifampicin resistant cases, and only 7 cases of inhA involvement conferring Isoniazid resistance in Rifampicin sensitive cases.
Genetic pattern analysis of these isolates were done by reversed hybridization technology using oligonucleotide probes covering the inhA promoter region covering codon 8 to 16 and katG region covering at the 315 codon.
inhA codes for the Enoyl-ACP reductase of the type II fatty acid synthase (FAS-II) system. The enzyme helps in the biosynthesis of mycolic acids, predominant component of mycobacterial cell walls [7].
Enoyl ACP Reductase Catalyzes the NADH-dependent reduction of the double bond of 2-trans-enoyl-[acyl-carrier protein], a fatty acid elongation step cycle of the FAS-II pathway [13]. It is seen the exclusive point Mutation of the 15th base pair promoter sequence of inhA that converts Cysteine residue to Threonine contributes the most among the inhA conferred resistance towards Isoniazid (Figure 1).
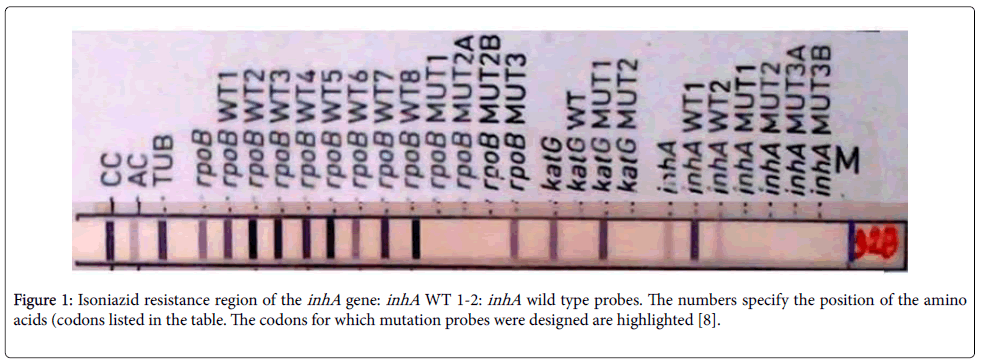
Figure 1:Isoniazid resistance region of the inhA gene: inhA WT 1-2: inhA wild type probes. The numbers specify the position of the amino acids (codons listed in the table. The codons for which mutation probes were designed are highlighted [8].
Involvement of katG and inhA with respect to rifampicin susceptibility
The presence of most common pattern was Presence of MUT1 with the absence of WT1. The pattern contributes most to the inhA dependent Isoniazid resistance isolates among retreatment cases. Percentage contribution of Band Patterns of Isoniazid resistance due to inhA.
For katG the most common pattern was seen as MUT1 (Pos) and WT (NEG) where a point mutations has occurred in codon 315 (Table 2). It has been observed that the inhA resistance pattern is seen in 11.7% and katG resistance is 90% among the Rifampicin resistant isolates however inhA resistance pattern is seen only in 1.1% and kat G in 17.8 % among the Rifampicin sensitive isolates isolated from the TB patients (Tables 3-6).
| Isoniazid inhA resistance pattern in rifampicin resistant cases n=442 (11.7%) | |||
|---|---|---|---|
| S.No. | Observation/pattern | Probe involved | Total cases detected |
| 1 | Resistance only due to absence of Wild Type band (s) with no positive Mutation band | WT1 | 8 (15%) |
| WT2 | 2 (3.8%) | ||
| WT1+WT2 | 2 (3.8%) | ||
| 2 | Resistance due to development of positive Mutation band (s) and absence of corresponding Wild Type band (s) | MUT1 (POS)/WT1 (NEG) | 35 (67.3%) |
| MUT2 (POS)/WT1 (NEG) | 4 (7.6%) | ||
| MUT3A (POS)/WT2 (NEG) | 0 | ||
| MUT3B (POS)/WT2 (NEG) | 0 | ||
| More than one MUT (POS) and NO WT developed | 0 | ||
| 3 | Resistance due to presence of Mutation band (s) whereas corresponding Wild Type band is also positive. | MUT1 (POS)/WT1 (POS) | 1 (1.9%) |
| MUT2 (POS)/WT1 (POS) | 0 | ||
| MUT3A (POS)/WT2 (POS) | 0 | ||
| MUT3B (POS)/WT2 (POS) | 0 | ||
| More than one MUT and WT (POS) | 0 | ||
Table 3: Patterns contributing to the resistance.
| Isoniazid katG resistance pattern in rifampicin resistant cases n=442 (90%) | |||
|---|---|---|---|
| S.No. | Observation/pattern | Probe involved | Total cases detected |
| 1 | Resistance only due to absence of Wild Type band (s) with no positive Mutation band | WT | 26 (6.3%) |
| 2 | Resistance due to development of positive Mutation band (s) and absence of corresponding Wild Type band (s) | MUT1 (POS)/WT (NEG) | 364 (88.7%) |
| MUT2 (POS)/WT (NEG) | 2 (0.48%) | ||
| MUT1+MUT2 (POS)/WT (NEG) | 0 | ||
| 3 | Resistance due to presence of Mutation band (s) whereas corresponding Wild Type band is also positive. | MUT1 (POS)/WT (POS) | 18 (4.3%) |
| MUT2 (POS)/WT (POS) | 0 | ||
| MUT1+MUT2 (POS)/WT (POS) | 0 | ||
Table 4: Patterns of katG contributing to the resistance in Rifampicin resistance cases.
| Isoniazid inhA resistance pattern in rifampicin sensitive cases n=627 (1.1%) | |||
|---|---|---|---|
| S.No. | Observation/Pattern | Probe Involved | Total Cases Detected |
| 1 | Resistance only due to absence of Wild Type band (s) with no positive Mutation band | WT1 | 0 |
| WT2 | 0 | ||
| WT1+WT2 | 1 | ||
| 2 | Resistance due to development of positive Mutation band (s) and absence of corresponding Wild Type band (s) | MUT1 (POS)/WT1 (NEG) | 5 |
| MUT2 (POS)/WT1 (NEG) | |||
| MUT3A (POS)/WT2 (NEG) | 0 | ||
| MUT3B (POS)/WT2 (NEG) | 0 | ||
| More than one MUT (POS) and NO WT developed | 0 | ||
| 3 | Resistance due to presence of Mutation band (s) whereas corresponding Wild Type band is also positive. | MUT1 (POS)/WT1 (POS) | 1 |
| MUT2 (POS)/WT1 (POS) | 0 | ||
| MUT3A (POS)/WT2 (POS) | 0 | ||
| MUT3B (POS)/WT2 (POS) | 0 | ||
| More than one MUT and WT (POS) | 0 | ||
Table 5: Patterns of inhA contributing to the resistance in Rifampicin sensitive cases.
| Isoniazid katG resistance pattern in rifampicin sensitive cases n=627 (17.8%) | |||
|---|---|---|---|
| S.No. | Observation/pattern | Probe involved | Total cases detected |
| 1 | Resistance only due to absence of Wild Type band (s) with no positive Mutation band | WT | 6 (5.3%) |
| 2 | Resistance due to development of positive Mutation band (s) and absence of corresponding Wild Type band (s) | MUT1 (POS)/WT (NEG) | 93 (83%) |
| MUT2 (POS)/WT (NEG) | 2 (1.7%) | ||
| MUT1+MUT2 (POS)/WT (NEG) | 0 | ||
| 3 | Resistance due to presence of Mutation band (s) whereas corresponding Wild Type band is also positive. | MUT1 (POS)/WT (POS) | 11 (9.8%) |
| MUT2 (POS)/WT (POS) | 0 | ||
| MUT1+MUT2 (POS)/WT (POS) | 0 | ||
Table 6: Patterns of katG contributing to the resistance in Rifampicin sensitive cases.
Here it is to be noted that MUT1 involves a 15th region of inhA promoter gene [8]. On analyzing the data for katG resistance pattern majority (88.7%) have resistance due to positive MUT 1 and negative WT 1 whereas 4.3% have Positive MUT1 and Positive WT 1 (Tables 3 and 4).
Among the inhA resistance pattern 67.3% has resistance due to positive MUT 1 and negative WT 1 whereas 1.9% has Positive MUT1 and Positive WT 1. It is known that inhA confers low resistance towards Isoniazid and it is found in 21% of the MDR cases [14]. In the study it was observed only 11.7% among the retreatment MDR cases had Isoniazid resistance due to mutation in the inhA.
The presence of the inhA induced resistance below the evaluated observation among the retreatment cases may prompt conversion of high level resistance due to katG owing to the long retreatment protocols due to dearth of availability of second line drugs before 2009.
The majority of the inh A resistance in the study occurred due to the mutations in the codon 15 and 16 of the inhA promoter gene. Sequencing would definitely reveal that whether any rare mutations prevail beyond this zone.
Out of the 442 cases 410 cases (92.7%) showed resistance to Isoniazid with the involvement of katG among rifampicin resistant cases however of the 627 Rifampicin sensitive cases 112 cases (17.8%) showing resistance to Isoniazid with the involvement of katG .
Isoniazid as a prophylaxis
It was seen that prophylaxis by Isoniazid for 36 months in HIV infected contacts was more effective than six months of prophylaxis [15]. The study by Martison et al., showed importance of combination drugs during prophylaxis [16].
Though some studies indicated long term prevention, the chance of not developing the disease in life time cannot be ruled out [17]. Preventive therapy is well tolerated in LTBI patients undergoing renal transplantation [18].
Based on the studies WHO recommend Isoniazid as a preventive therapy for HIV infected LTBI [19]. There are publications to corroborate the efficacies of the drug when used as a prophylaxis. But most of the studies are based on the clinical outcomes and results of the baseline diagnostic tests with varied analytical sensitivities. The exact quantum of infection with the exact strain responding to the treatment is lacking in all most all the studies thereby raising a question on the rationale of its use. The study explores the point and periodic prevalence of the Isoniazid resistant strains. The pattern of resistance is shown owing to their point mutation [19-22].
The band Pattern analysis for inhA related mutations showed the majority of the mutations to be occurring at the 15th codon of the inhA promoter region. In only one rare occasion presence of both mutant and wild type segments are found. This might be a case of mixed population. The lower percentage of inhA dependent mutations with respect to the evaluated studies may be a result of the prolonged retreatment schedules. The study was done among the 442 Rifampicin resistant isolates recovered from retreatment cases in the year 2012 as well as 627 from newly diagnosed Rifampicin sensitive cases from a period of 2013-2016.
These cases were on retreatment schedule since long as no second line drugs were available before 2012. A pear pressure for prolonged retreatment regimen might have converted several of low dose resistant strains to high dose resistant strains.
The study revealed an alarming 92.7% percentage of resistance among the Rifampicin resistant cases. Moreover katG induced resistance was found to be on the rise 87.5 Percentage which suggested the increasing resistance towards Isoniazid. India accounts for an Annual rate of transmitting infection 1.5, failure of preventive treatment adherence would worsen the situation rendering the increase in Isoniazid resistance among the population.
Out of 442 cases 410 cases showed Isoniazid resistance. Out of which resistance due to inhA was seen in 32 cases, 19 cases showed resistance due to mutations in both inhA as well as katG. 359 cases accounting to 87.5% showed exclusive involvement of katG . As it is evident that katG confers higher degree of Isoniazid resistance, the higher percentage of katG induced Isoniazid resistance among the Rifampicin resistant strains depicts the spread of higher degree of Isoniazid resistance being the driver for Rifampicin resistance. A similar study for Rifampicin resistance is carried over showing conversion of sensitive strain to resistant ones among heterogeneous strains for patients who are put on anti-tubercular drugs.
Initially it was thought that in Multi Drug Resistant-Tuberculosis, exposure to both Isoniazid and Rifampicin results in the mutations that confer resistance to each drug. However, in vitro analysis suggested that pre-existing INH resistance allele has a role in influencing the subsequent rpoB mutations. This has been corroborated in our study when around 92.7 percent of the Rifampicin resistant strains shown to have an existing high degree of Isoniazid resistance by katG . Seconding the observations drug resistant clinical Mycobacterium tuberculosis isolates have shown that specific INH resistance allele are more frequently associated with resistances to other drugs.
The question remains how effective would be the INH prophylaxis in pediatric age group contacts of the cases in this scenario. The rise of the high level of resistance may suggest the irrational empirical use of the drug as a prophylaxis.
The Preventive treatment of Tub by a drug which accounts for substantial spread of its resistance needs further introspection. The efficacies studies are based on duration of ten to fifteen years. The chances of disease development in a lifetime among the subjects given by the conventional means of preventive needs are to be reviewed. A wise rationale would be administering a drug whose sensitivity is prevalent in most of the isolated strains from the source cases. Preventive therapy based on the Drug sensitivity of the source cases would be more judicious to fight out the latent infections among the children and contacts.
Due to high Isoniazid resistance in cases where Rifampicin resistance is present the Isoniazid prophylaxis in contacts is even worsening the situation as base line 92.7% of Isoniazid resistance is noted through katG in our extensive study.
To achieve the target of control of Tuberculosis cases globally not only treatment to be focused on Universal Drug Sensitivity Testing and early initiation of treatment based on the sensitivity, but also care to be taken for prophylaxis of drugs to contacts of established cases, and if resource permits selection of drugs for prophylaxis should be also Drug sensitivity guided.
We are thankful to all Medical officers and Technical officers, RNTCP West Bengal, India for providing technical inputs and all patients and staffs contributing for their dedication.
The Authors do not have any Conflict of interest
Citation: Das PK,Ganguly SB, Mandal B (2019) Detection of Genetic mutations in inhA and katG for Isoniazid and its Association with Rifampicin Resistance in Tuberculosis Confirmed by Line Probe Assay-its Rationality for Isoniazid Prophylaxis Empirical or DST Guided. Mycobact Dis 9:274. doi:10.4172/2161-1068.1000274
Received: 18-Feb-2019 Accepted: 15-Mar-2019 Published: 25-Mar-2019 , DOI: 10.35248/2161-1068.19.9.274
Copyright: © 2019 Das PK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.