Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2019)Volume 11, Issue 1
In this study, we tested for the antiviral potentials of derivatized extracts from two plants Aframomium melegute K. Schum. and Vernonia amygdalina Delile against atypical Fowl pox virus (FPV Kabete). Preliminary study on derivatized extracts from the plants showed that A. melegute extract contain two abundant phenols (benzaldehyde-3-hydroxy-4methoxy and butan-2-one-4-(3-hydroxy-2-methoxyphenyl) while the V. amygdalina extract contained Phytol and a nucleoside analogue Methyl-2-O-benzyl-d-arabinofuranoside as most abundant compounds. We determined the prophylactic and therapeutic efficacy of the derivatized extracts against the FPV in Embryonated Chick Eggs (ECEs) and also linked their antiviral properties to the activity of the most abundant compounds they contain. Results showed that the extracts had strong therapeutic and prophylactic efficacy against FPV. Summarily, the extract from A. melegueta had stronger prophylactic efficacy against the FPV with an inhibition concentration (IC50) of 159.49 ± 2.16 µm, it reduced the mortality of embryos (from 21.46 ± 2.31% to 6.89 ± 0.29%), diminished the FPV Log10EID50 titer from 3.86 to 2.78 and its percentage viral reduction index for prophylactic assay was at 91.7%. However, the V. amygdalina extract showed better therapeutic potential with inhibition concentration (IC50) of 179.90 ± 2.74 µm, it strongly reduced the embryo mortality (from 21.46 ± 2.31% to 7.72 ± 0.34%) and the FPV Log10EID50 titer diminished from 3.86 to 3.08 while it had a viral percentage reduction index of 83.4% for the therapeutic assay. This study demonstrated the antiviral efficacy of derivatized extracts from the two test plants against FPV and that compounds contained in the extracts can serve as molecular leads in subsequent study for biosynthesis of novel antipoxvirus inhibitors.
Phenols; Nucleoside analogues; Antiviral potential; Fowl pox virus; Phytol; ECEs
The development of biology, genetics, medicine, pharmacy at the end of XX century, bring along cloning of mammals, made the map of the human genome, genetic investigation and gene therapy reach new successes, assisted reproduction to become available procedure
Fowl pox is an important disease of poultry and other domestic birds caused by fowl pox virus, a non-segmented DNA virus. The outbreak of the disease in poultry leads to important economic loses to the owners. Nyaga et al. reported the occurrence of an “atypical” Fowlpox virus (FPV) strain in Kabete, Kenya, and the virus strain caused heightened epithelial proliferation, mild paralysis, endotheliosis and lympho-proliferative damages of matured birds [1]. Other studies in recent years affirmed that the atypical FPV might cause mild infections in man [2]. More so, Zhao et al. [3] characterized the atypical FPV whole genome and confirmed that the atypical FPV (Kabete) strain reported by Nyaga et al. [1] had reticulo-endotheliosis virus (REV) genes integrated into its whole genome. Hence, these reports underscore the need for alternative trials of natural products derived from antiviral compounds to combat the FPV strain since live attenuated vaccines might not confer absolute immunity.
The recent strides made in pharmacopeia delineated the potency of phyto-compounds such as alkanols, nucleoside base analogues and phenols as drug leads against re-emerging infectious strains of viruses. Scientists regard nucleoside analogues as suitable antiviral drug leads since they block the replication of many enveloped DNA viruses in living tissues [4] or actively hinder the entry of viruses into living tissues [1]. In his holistic study, Nittya and Suaresh [5] suggested that the plant V. amygdalina Del; might possess antiviral compounds since local respondents in parts of Western Africa use it against measles invasion while another recent report from Faeji et al. [6] showed that extract from the plant have antiviral activity against Newcastle Disease Virus strain (NDV N1E). Moreover, Aframomium melegueta K. Schum. is another plant with a rich source of phenols and past findings showed the plant possess stellar antimicrobial efficacy, although, there exist scant data to demonstrate its efficacy against viruses [7]. Recent studies involving the testing of antiviral activity of plants have sought to use derivatized forms of plant extracts containing known organic compounds in verified quantities [4]. This has helped scientists to be more precise as regards dose dependent analysis of antiviral drug compounds and more importantly, to encourage compound biosynthesis.
In this study, we tested the antiviral efficacy of derivatized extracts from A. melegueta and V. amygdalina against an atypical Fowlpox virus strain (FPV Kabete). Preliminary studies on the derivatized extracts already showed that the V. amygdalina extract contain two abundant compounds (Phytol and a nucleoside analogue Methyl-2-O-benzyl-d-arabinofuranoside) while the A. melegueta extract contain two abundant phenols (benzaldehyde-3- hydroxy-4-methoxy and butan-2-one-4-(3-hydroxy-2- methoxyphenyl). We determined the multiple therapeutic and prophylactic effects of these compounds in derivatized extracts from the two plants against FPV on Embryo Chick Egg cultures (ECEs) and linked the possible mechanisms of action responsible for the antiviral efficacy exhibited by these compounds in the extracts.
A brief on the major organic compounds present by mass in derivatized extracts
Extracts from the test plants A. melegueta and V. amygdalina were obtained by adopting procedures described by Huang et al. [8]. After extraction process, protocols native to the Agilent Turbo Mass Spectrophotometer 7890A GC with chemstation NIST was adopted for derivatization of the extracts obtained [9]. Identification and quantification of the major organic compounds in the derivatized extracts were carried out using Agilent Turbo Mass Spectrophotometer 7890A GC with chemstation NIST for Gas Chromatography-Mass Spectrometry (GC-MS) analysis. The two concentrates from the plants have been analyzed and clarified prior to antiviral assay. Full details of these procedures are already in a publication (short communication) currently undergoing peer review but summarized in Figures 1 and 2.
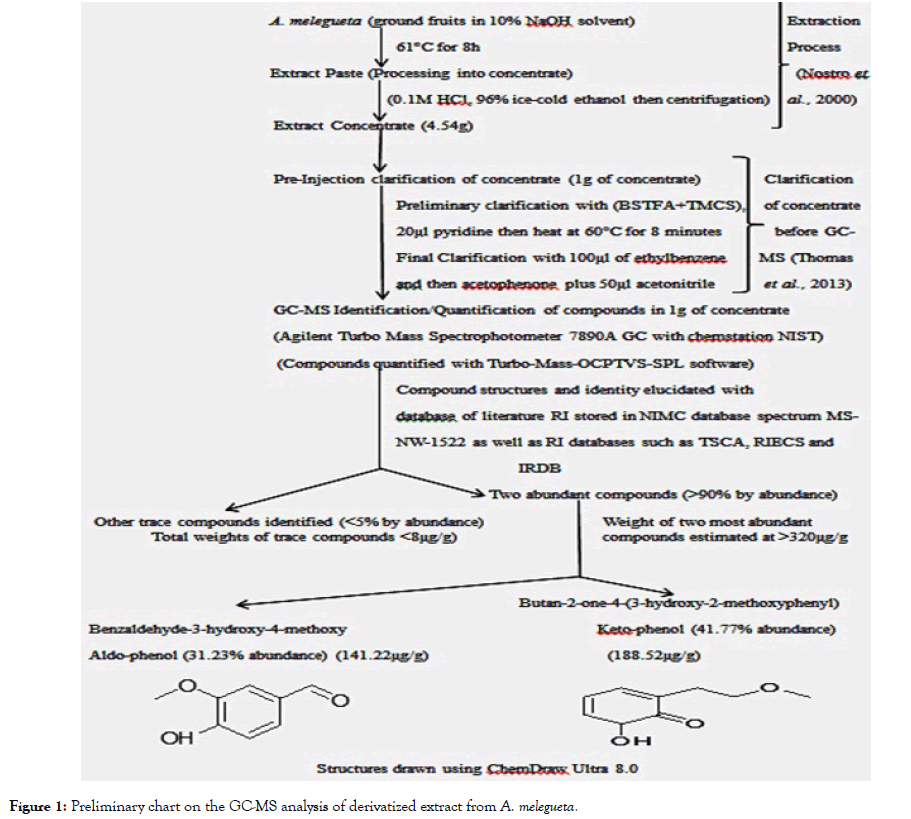
Figure 1: Preliminary chart on the GC-MS analysis of derivatized extract from A. melegueta.
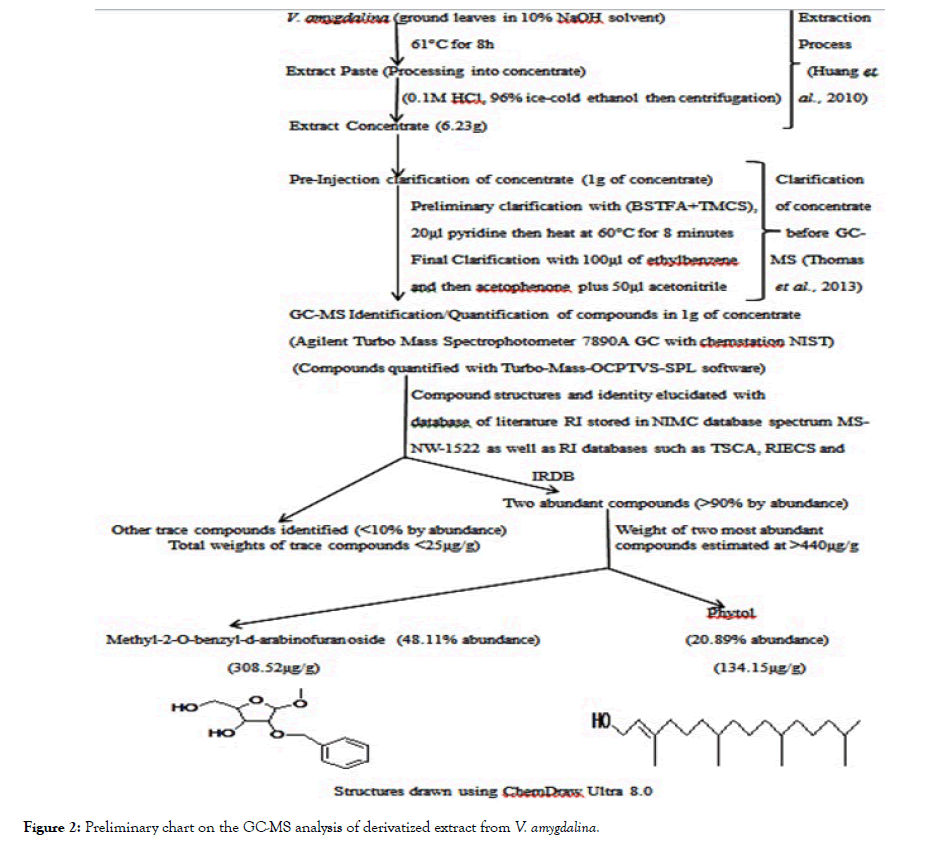
Figure 2: Preliminary chart on the GC-MS analysis of derivatized extract from V. amygdalina.
Virus source and Egg Cultures (ECEs)
The atypical strain of Fowlpox virus (FPV Kabete) used in this study was obtained from the Viral Vaccines Production Division of National Veterinary Research Institute (NVRI), Vom and Embryonated Chick Eggs (ECEs) aged 12 days were from the poultry division of NVRI, Vom, Nigeria. Before use, the embryonated eggs were candled to check for viability. The viable eggs were punctured at the chorio-allantoic region and at a region about 3 mm below the air sac, using pressure from a vacuum pump (Z844-V Weitzler, Germany) to create artificial space and collapse the chorioallantoic membrane for inoculation of the extracts into Chorioallantoic membrane (CAM). The in-ovo extract toxicity assay, determination of embryo infectious dose (EID50), inhibitory and therapeutic effects of derivatized extracts using ECEs were carried out at viral research division of the NVRI, Vom, Nigeria.
Reconstitution of derivatized extracts before antiviral assay
A 50 mm stock solution of the derivatized extracts were prepared by reconstituting 0.5 g of the each extract obtained after the GC-MS into 10 ml of the diluent consisting of Penicillin, Streptomycin, Gentamycin, and Amphotericin B (PSGA X5) adjusted to pH 7.2. The resulting solutions were filtered using 0.23 μ membrane filters via suction pressure filtration into sterile vials and stored at -4°C until needed.
Acute toxicity assay of derivatized extracts on ECEs
The toxic effects of derivatized extracts on chick embryos were tested to determine its minimum toxic concentration in 13 days old ECEs. The 13 days old ECEs were divided into four groups of five ECEs per group. The ECEs carried labels as appropriate in biosafety cabinets and acclimatized ECEs in humified incubator (Schumboldt CR-8V CO2 pression Incubator). From the 50 mm stock earlier prepared, three different dilutions of the derivatized extracts (450 μm, 300 μm, and 150 μm) were obtained. The eggs were swabbed with 70% ethanol and a 0.2 ml of each dilution was separately inoculated into the allantoic cavity of labeled ECEs in their respective groups while the control groups received 0.2 ml PBS+PSGA X5 [6]. The inoculation sites were sealed with paraffin wax and the eggs were incubated for 120 hours at 37 ± 1°C. The eggs were candled daily and dead embryos were removed. At the end of the incubation period, embryos were harvested and the toxic effect of the various dilutions of the concentrate on the embryos were determined by examining them for hemorrhages and evaluating percentage mortality of embryos in each group [6].
Determination of Initial Egg Infectious Dose (EID50) of FPV
The determination of the mean embryo infectious dose (EID50) of FPV Kabete was according to the methods of Nyaga et al. [1] and Young et al. [10]. A 0.9 ml of PBS was dispensed into labeled sterile tubes and a 0.1 ml of the virus stock was used to prepare serial dilutions of 10-1 to 10-7. The 13 days old ECEs were divided into seven groups of five Eggs per group and labeled appropriately under biosafety cabinets. The eggs were swabbed with 70% ethanol and inoculated ECEs with 0.1 ml of each virus dilution via the CAM. Inoculation sites were sealed with paraffin wax and eggs were incubated for 120 hours at 37 ± 1°C. The inoculated eggs were candled daily to check for mortality. At the end of the incubation period, the chorioallantoic membranes (CAMs) of surviving ECEs were harvested aseptically and examined for pock lesions. The Spearman-Karber formula (1931) was used to calculate the Egg infectious dose 50 (EID50) of the virus.
Antiviral assays of derivatized extracts against FPV
The antiviral efficacy of extracts against FPV using ECEs was according to the methods of Nyaga et al. [1] and Faeji et al. [6]. From the toxicity assays, the minimum toxic concentration threshold of 300 μm was used and a 100 EID50 virus stock solution was prepared. The 13 days old ECEs were divided into six test groups of fifteen ECEs each while the control groups had six ECEs. The eggs were labeled as appropriate and sites of inoculation were swabbed with 70% ethanol under biosafety cabinets. To perform the inhibition assay, a 0.1 ml/300 μm of each extract was first inoculated into the ECEs via the CAM infection route and incubated for at 37°C for 90 minutes for the concentrate to adsorb to the CAM. At the end of 90 minutes, 100 EID50/0.1 ml FPV was inoculated into the eggs. The eggs were sealed with paraffin wax and incubated at 37°C for 120 hour. The eggs were candled daily and dead embryos were removed. To perform the therapeutic assay, a 100 EID50/0.1 ml FPV was first inoculated into the eggs via the CAM and incubated for 90 minutes for virus adsorption. At the end of 90 minutes 0.1 ml/300 μm of each extract was inoculated into the eggs. The eggs were sealed with paraffin wax and incubated at 37°C for 120 hours. The eggs were candled daily and dead embryos were removed. At the end of the incubation periods, CAM and embryos were aseptically harvested from the surviving eggs. The embryos were examined for deformities and the mortality rates of the chick embryos were recorded for each group. The dose dependent concentration (IC50) of each derivatized extract against FPV was also obtained for therapeutic and prophylactic assays.
Determination of final FPV EID50 titer after treatment assays
The CAMs from the treatment groups were harvested separately into sterile cruise vessels and CAMs were soaked in PBS for 4 hours. The soaked CAMs were crushed and homogenized with a pestle. The marshed CAMs were centrifuged at 5000 rpm for 30 minutes to obtain homogenates of the CAMs. From this, a 20% of the homogenate was prepared as the virus stock for final FPV EID50 determination.
The same procedures earlier described for the initial EID50 determination as described by Nyaga et al. [1] and Young et al. [10] was repeated and the final EID50 FPV titer was determined using the Spearman-Karber formula (1931).
Data analysis
Different statistical tools in GraphPad Prism 5.0 were used to achieve all data analyses in this study. The reduction index of FPV EID50 titer (initial and final) after treatments assays, the activity curves of extracts against FPV EID50 and the average mortality of eggs across different treatment groups were analyzed.
Furthermore, the antiviral activity of derivatized extracts by dose dependent 50% inhibition concentration (IC50) was determined using non-linear regression analyses.
Toxicity of extracts in ECEs
The derivatized extracts contained different compounds that have been characterized in our preliminary studies. However, the major compounds had >70% abundance by weight after compound recovery by GC-MS in the derivatized extracts from the two plants. The two compounds most abundant in V. amygdalina extract were Phytol with 20.89% abundance (a recovery index of 134.15 μg per 1 g) and the nucleoside analogue Methyl-2-O-benzyl-d-arabinofuranoside with 48.11% abundance (recovery index of 308.52 μg per 1g) while two compounds most abundant in A. melegueta extract were the aldophenol benzaldehyde-3-hydroxy-4-methoxy with 31.23% abundance (a recovery index of 141.22 μg per 1 g) and the ketophenol butan-2-one-4-(3-hydroxy-2-methoxyphenyl) with 41.77% abundance (recovery index of 188.52 μg per 1 g). The information on the toxicity assay of the extracts from the plants is in Table 1. Evaluations on the probable toxicity of the extracts in ECEs were done by juxtaposing the mortality rates and occurrence of deformities in the embryos with the control groups. More so, the mortalities across the different dilutions of the derivatized extracts from both plants were also compared with the standard index set for atypical Fowl pox virus.
| Test Plants | Test Groups (Extract dilutions) | Mean Mortality in test groups (%) |
Mean Mortality in Control (Ct. G) (%) | Mean Mortality threshold set for ECE toxicity (%) | Deformities in ECEs (Test groups) | Deformities in ECEs (Control Ct. G) |
|---|---|---|---|---|---|---|
| V. amygdalina | ED1 | 22.22 ± 2.05d | 00 ± 00a | 30.00 ± 1.00e | Absent | Absent |
| ED2 | 12.59 ± 2.06bc | 00 ± 00a | 30.00 ± 1.00e | Absent | Absent | |
| ED3 | 9.40 ± 1.19b | 00 ± 00a | 30.00 ± 1.00e | Absent | Absent | |
| A. melegueta | ED1 | 20.37 ± 1.40d | 00 ± 00a | 30.00 ± 1.00e | Absent | Absent |
| ED2 | 7.72 ± 0.34b | 00 ± 00a | 30.00 ± 1.00e | Absent | Absent | |
| ED3 | 5.34 ± 1.67b | 00 ± 00a | 30.00 ± 1.00e | Absent | Absent |
The second dilution (ED2) was chosen as the concentration to use for the antiviral assays in both plants
Keys: ED1-450 µm dilution, ED2-300 µm dilution, ED3-150 µm dilution, Ct. G-Control group having no treatment with extracts, values with same superscript have no significant difference at p ≤ 0.05.
Table 1: The mean mortality of ECEs with respect to toxicity of derivatized extracts.
The mean Embryo Infectious Dose (EID50) of FPV in ECEs
The Table 2 shows how the EID50 of FPV was determined in ECEs using the Spearman-Karber formula. The result suggests that the atypical FPV has high virulence in ECEs since comparably lower dilutions of the FPV stock still had mortalities across the treatment groups observed in this study.
| Dilutions (in factors of 10) | Pock Positive (P) | Pock Negative (N) | Cumulative Positive (Cp) | Cumulative Negative (Cn) | Overall positive Cumulative (%) | Extrapolation |
|---|---|---|---|---|---|---|
| A | 5 | 0 | 13 | 0 | 100 | -- |
| B | 4 | 1 | 8 | 0 | 80 | -- |
| C | 3 | 2 | 4 | 2 | 66.7 | P.D. |
| D | 1 | 4 | 1 | 6 | 14.29 | |
| E | 0 | 5 | 0 | 11 | 0 | -- |
| F | 0 | 5 | 0 | 16 | 0 | -- |
| G | 0 | 5 | 0 | 21 | 0 | -- |
Keys: A: Dilution 1, B: Dilution 2, C: Dilution 3, D: Dilution 4, E: Dilution 5, F: Dilution 6, G: Dilution 7, P.D.: Partial difference. Authors harvested all the ECEs and observed for pock formation after incubation period of 120 hours.
Table 2: Egg Infectious Dose determination (EID50 Determination).
The EID50 was determined using the Spearman-Karber formula of LogEID50={X+½(logd) – {(ΣB/n)+1+P.D.}
Where
X is the range of all dilutions used,
d is the square of dilution factor constant (10),
B is the negative cumulative below 50%,
n is dilution factor below 50% and
P.D. is the partial difference.
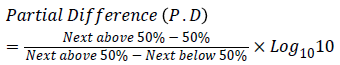
{66.7 − 50/66.7 − 14.29 = 16.7/52.4 = 0.32
Hence, Partial Difference (P.D)=0.32.
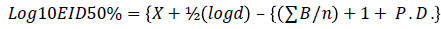
From the table, X=6, d=100, B=11, P.D.=0.32.
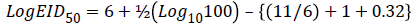
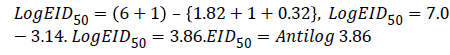
Hence, EID50=7244.4 ml-1
Therefore Viral Titer EID50=Log103.86/0.1 ml of FPV or 0.01: 72.44(1/7244.4 ml)
Evaluation of antiviral efficacy of derivatized extracts against atypical FPV
The results in Tables 3 and 4 showed that the extracts drastically reduced the lethality of the atypical FPV in ECEs as evidenced in the mortality rates. The tables also showed that the extract’s activity against the lethality of the FPV depends on the type of treatments used whether prophylactic or therapeutic. In Table 3, the A. melegueta extract reduced the lethality of the FPV more in the prophylactic treatment (reduction of mortality from 21.46 ± 2.31% to 6.89 ± 0.29%) while Table 4, shows that the V. amygdalina extract reduced the lethality of the virus more in the therapeutic treatment (mortality was reduced from 25.09 ± 2.14% to 7.72 ± 0.34%) (Table 5). In the Figures 3 and 4, the virus titer curves of the extracts against the FPV virus showed the reduction in the FPV viral activity caused by the administration of the extracts both in prophylactic and therapeutic assays.
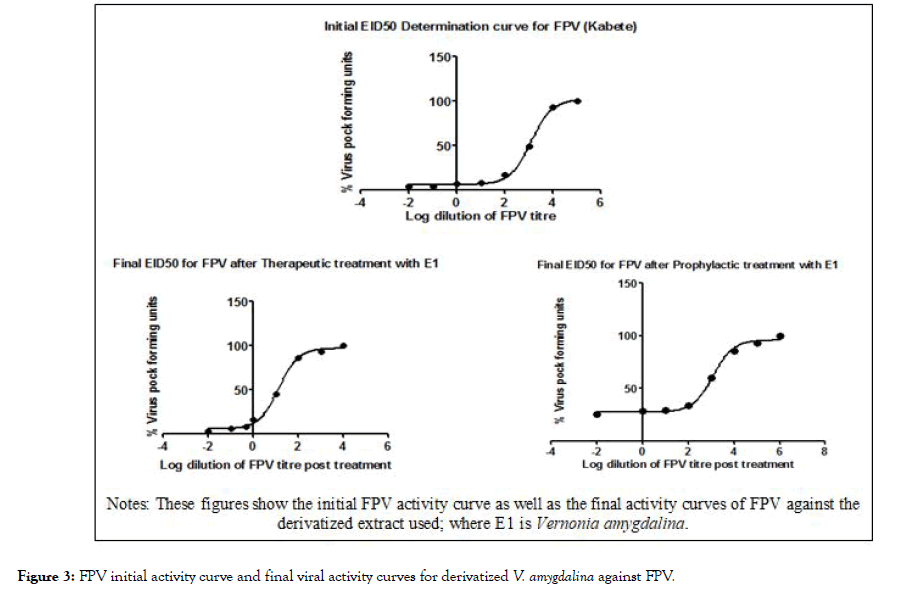
Figure 3: FPV initial activity curve and final viral activity curves for derivatized V. amygdalina against FPV.
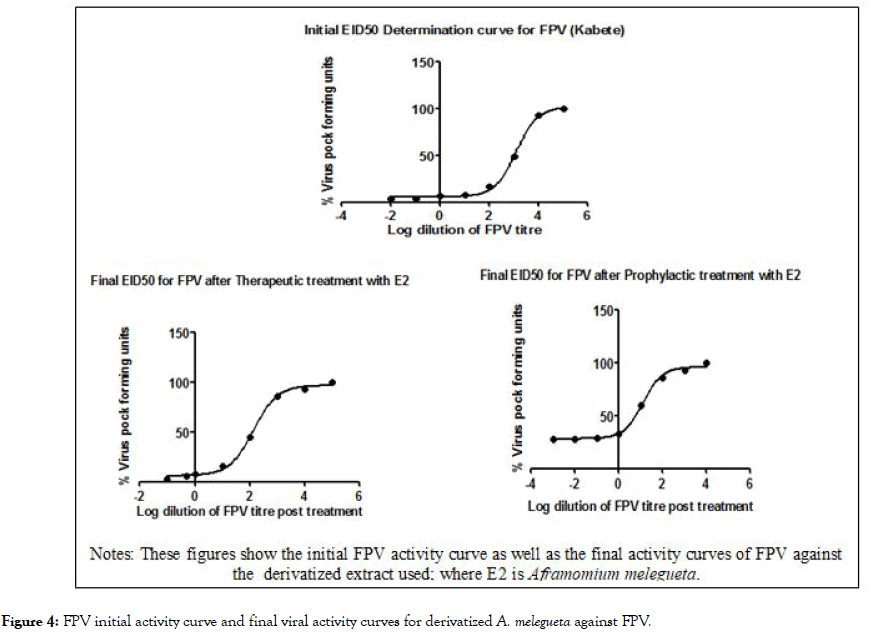
Figure 4: FPV initial activity curve and final viral activity curves for derivatized A. melegueta against FPV.
| Test Plant | Treatment Types |
Test Groups | Mean Mortality in test groups (%) |
Mean mortality virus control group (G2) (%) | Mean Mortality threshold set for antiviral assays (%) | Deformities in ECEs (Test groups) | Deformities in ECEs (virus control G2) |
|---|---|---|---|---|---|---|---|
| Aframomium Melegueta | Therapeutic Assay | G1 | 10.47 ± 1.46c | 21.46 ± 2.31d | 50.00 ± 1.00e | Present | Present |
| G3 | 3.39 ± 0.57ab | 21.46 ± 2.31d | 50.00 ± 1.00e | Absent | Present | ||
| G4 | 00 ± 00a | 21.46 ± 2.31d | 50.00 ± 1.00e | Absent | Present | ||
| Prophylactic Assay | G1 | 6.89 ± 0.29b | 21.46 ± 2.31d | 50.00 ±1.00e | Absent | Present | |
| G3 | 3.39 ± 0.57ab | 21.46 ± 2.31d | 50.00±1.00e | Absent | Present | ||
| G4 | 00 ± 00a | 21.46 ± 2.31d | 50.00±1.00e | Absent | Present |
The A. meleguetaextract reduced the embryo mortality in both assays, but the extract was more potent for the prophylactic inhibition rather than therapy
Table 3: Reduction of mortality rates in ECEs by derivatized A. melegueta extract against FPV.
| Test Plant | Treatment Types |
Test Groups | Mean Mortality in test groups (%) |
Mean mortality virus control group (G2) (%) | Mean Mortality threshold set for antiviral assays (%) | Deformities in ECEs (Test groups) | Deformities in ECEs (virus control G2) |
|---|---|---|---|---|---|---|---|
| V. amygdalina | Therapeutic Assay | G1 | 7.72 ± 0.34b | 25.09 ± 2.14d | 50.00 ± 1.00e | Absent | Present |
| G3 | 3.95 ± 0.62ab | 25.09 ± 2.14d | 50.00 ± 1.00e | Absent | Present | ||
| G4 | 00 ± 00a | 25.09 ± 2.14d | 50.00 ± 1.00e | Absent | Present | ||
| Prophylactic Assay |
G1 | 12.72 ± 1.22c | 25.09 ± 2.14d | 50.00 ± 1.00e | Present | Present | |
| G3 | 3.95 ± 0.62ab | 25.09 ± 2.14d | 50.00 ± 1.00e | Absent | Present | ||
| G4 | 00 ± 00a | 25.09 ± 2.14d | 50.00 ± 1.00e | Absent | Present |
The V. amygdalinaextract reduced the embryo mortality rate more for the therapeutic assay rather than for prophylactic inhibition
Keys: G1-test group with 300 µm concentrate plus 100EID50 FPV, G3-Virus control with 100EID50 FPV alone, G3-concentrate control with 300 µm concentrate alone, G4-ECE control with no treatment.
Table 4: Reduction of mortality rates in ECEs by derivatized V. amygdalina extract against FPV.
| Test Plant | Treatment Types | Initial FPV Titre | Inhibition Concentration of extract concentrate (IC50) μm | Final FPV Titre | Reduction Index of extract concentrate (%) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Log10X | Equivalent volume (ml-1) |
Log10X | Equivalent volume (ml-1) |
|||||||
| A. melegueta | Prophylactic | 3.86 | 7244.40 | 159.49 ± 2.16 | 2.78 | 601.30 | 91.70 | |||
| Therapeutic | 3.86 | 7244.40 | 216.85 ± 4.18 | 3.34 | 2209.50 | 69.50 | ||||
| The results above showed that A. meleguetaextract was more potent for inhibition of atypical FPV while it was less potent for therapy of FPV infections | ||||||||||
| V. amygdalina | Prophylactic | 3.86 | 7244.40 | 240.50 ± 4.68 | 3.45 | 2818.10 | 61.10 | |||
| Therapeutic | 3.86 | 7244.40 | 179.90 ± 2.74 | 3.08 | 1202.60 | 83.40 | ||||
These data shows that V. amgydalinaextract was more potent for therapy of atypical FPV while it was less potent for prophylactic inhibition of FPV infections
Table 5: Viral Reduction potential and dose dependent index of derivatized extracts from test plants.
Dose dependent activity of derivatized extracts against atypical FPV
The summary of the dose dependent antiviral efficacy of the derivatized extracts from the two test plants against the atypical FPV for both treatment assays (prophylactic and therapeutic) are in Table 4. The Table 4 shows have the percentage reduction index for A. melegueta extract (69.5% viral reduction for therapeutic assay, 91.7% viral reduction for prophylactic assay) and the percentage reduction for V. amygdalina (83.4% viral reduction for therapeutic assay, 61.1% reduction for prophylactic assay). Finally, the Table 4 also showed the effective dose for the derivatized extracts from the two plants. The A. melegueta extract had a better dose dependent average (IC50 of 159.49 ± 2.14 μm for prophylactic and IC50 of 216.85 ± 4.18 μm for therapeutic) compared to that of V. amygdalina (IC50 of 179.90 ± 2.74 μm for therapeutic assay and IC50 of 240.50 ± 4.68 μm for the prophylactic assay).
The most abundant compounds in the derivatized extracts [benzaldehyde-3-hydroxy-4-methoxy and butan-2-one-4-(3- hydroxy-2-methoxyphenyl)] for A. melegueta and [furan-based nucleoside analogue Methyl-2-O-benzyl-d-arabinofuranoside and the long chain alcohol Phytol] for V. amygdalina might have some degrees of toxicity on embryos in the ECEs (Table 1). Several reasons may account for the toxic effects of the extracts on the chick embryos. Jena et al. showed that a lethal dose (>1000 μm) of some nucleoside analogues triggers hepatic steatosis in hepatocytes of Wister albino rats eventually resulting in weight reduction of the rats [11]. The study also attributed tissue genotoxic damage to the overdose of nucleoside analogues since they are weak substrates of host tissue DNA polymerases.
Meanwhile, Islam et al. showed by his findings that phytol at concentrations above 600 μm form nano-emulsions in young tissues (artem salina model) thereby blocking kinase dependent inhibitors in such tissues [12]. Likewise, studies focusing on the dose dependent pharmacology of phenolic compounds have highlighted some of their potential toxic responses in living tissues. A study by Tapeiro et al. showed that some tissue functions become impaired at concentrations above 700 μm of plant phenols [13]. The study also attributed tissue surface prooxidation to phenolic overdose in Chick hamster cultures. Furthermore, Manach et al. posited that phenols might form inhibition complexes that hinder chemical factors involved in early prophase stage of mitosis by blocking protease dependent activators, leading to toxicity [14].
The mortalities associated with infections of poxviruses in tissues (ECEs) are generally low given the precedence of recent research. Singh and Tripathy explained this in citing most early reports in the last century of research focused on poxviruses and their lethality in living tissues [2]. However, the same study explained that the atypical FPV first reported in Kabete, Kenya has increased virulence and higher the average mortality than those observed in many earlier findings. As evident in the results (Table 2, Figures 3 and 4), the FPV activity and initial/ final FPV EID50 threshold showed higher virulence than what was expected on the average. This affirmed why the prospect of live attenuated vaccine production for the atypical FPV was quite infeasible in Zhao et al. [3]. The results (Figures 3 and 4) also showed how the antiviral efficacy of the extracts was since the replication and infection of FPV starts from the epithelial tissues of the ECEs resulting in virions multiplication and deformities of ECE embryos.
The strong antiviral efficacy of the extracts might be due to several cumulative factors. The phenols in the A. melegueta extract reacts directly with FPV lipid envelope and partially alter its recognition molecules [15,16]. however recently described how phenols ionize surface receptors on host tissues to cause reduction in the ability of viruses to make direct attachment to host tissues. This cause ligand induced non-fusogenic dissolution of viral membranes; perforating the FPV lipid bilayer envelope [16]. Since the A. melegueta extract contained two phenols in abundance, it follows that the phenols provide enough ions into cell cytoplasm, which will weaken the action of the FPV decapping enzymes D9 and D10 reducing the ability of the virus to replicate effectively [16]. These mechanisms involved in the antagonistic properties of the compounds in the A. melegueta extract ensures that the FPV is deactivated even before it can attach to the host tissues. This strongly explains why in the results, the A. melegueta extract had better prophylactic efficacy than the V. amygdalina extract.
However a study by Reaves et al. showed that phytol one of the compounds of abundance in the V. amygdalina extract cause production of weakened virions since the -OH end of phytol can de-hydrolyse the loose phosphate complexes of the viral DNA fragments involved in viral genome replication [15]. The phytol also weakens the recognition potential of FPV DNA polymerase, which then causes ineffective replication of virions effectively terminating the viral cycle. Alternatively, the V. amygdalina extract contain the furan-based nucleoside analogue Methyl-2-Obenzyl- d-arabinofuranoside; Hofman and Nelson showed that nucleoside analogues act as substrate mimics for early and late transcription promoter (TpV1 and TpV5) of the FPV thereby blocking effective viral replication in living tissues [17]. Furthermore, the benzyl-group attached to the furan-based nucleoside in the V. amygdalina extract affords it an advantage of mimicking standard purine bases without losing its toxicity after contact with the FPV concoctamer complex during late translation [15]. In synchrony, the ways by which the abundant compounds of the V. amygdalina extract exert antiviral effects of against the FPV shows that the compounds act better in a late phase of the viral cycle when infection has already been established. This accounts for why the V. amygdalina extract showed more therapeutic efficacy than the A. melegueta extract in the results. This study is limited majorly to the mechanisms by which the test plants act against the atypical FPV in ECEs alone, hence further studies are recommended [18,19].
The findings of this current study indicate that the compounds butan-2-1-4-(3-hydroxy-2-methoxyphenyl), benzaldehyde-3- hydroxy-4-methoxy, phytol and Methyl-2-O-benzyl-darabinofuranoside in the derivatized extracts from both plants may serve as molecular leads in subsequent study for development of new anti-poxvirus inhibitors in living tissues.
There authors declared no conflict of interests.
We would like to acknowledge the technical support offered by the entire staff of the viral research division, viral vaccines production unit and poultry division of National Veterinary Research Institute, Vom, Nigeria.
The authors received funding support from the Nigerian Tertiary Education Trust Fund (TETFund) for this research (Grant ID VCPU/TETFund/155).
Citation: Oladunmoye MK, Afolami OI, Oladejo BO, Amoo IA, Osho BI (2019) Derivatized Extracts from Aframomium melegueta K. Schum. and Vernonia amygdalina Delile Contain Organic Compounds that Showed Antiviral Effects against Atypical Fowl Pox Virus (FPV Kabete). J Antivir Antiretrovir. 11:181. doi: 10.4172/1948-5964.1000181
Received: 12-Mar-2019 Accepted: 21-Mar-2019 Published: 28-Mar-2019
Copyright: © 2019 Oladunmoye MK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.