Immunome Research
Open Access
ISSN: 1745-7580
ISSN: 1745-7580
Research Article - (2025)Volume 21, Issue 1
Purpose: Mitotic spindle tissue protein 2A (MZT2A) has the properties of the core subunit of the γ-Tubulin Ring Complex (γ-TuRC) and is involved in mediating the formation of bipolar spindles in mitosis. The lack of MZT2A will weaken the nucleation activity of Microtubule (MT) and slow down the rate of cell proliferation. Although there is evidence that the expression of MZT2A plays a crucial role in the development of some tumors, there is still no research on MZT2A in pan-cancer. Therefore, we aim to explore the prognostic value of MZT2A in a variety of cancer types and to study its potential immune function.
Methods: This study was conducted with datasets from The Cancer Genome Atlas, the Cancer Cell Lineage Encyclopedia, Genotype Tissue Expression and the Human Protein Atlas. Bioinformatic methods were used to analyse the correlation between MZT2A expression levels in different tumours and survival prognosis, immune infiltration, immune checkpoint genes, immune regulatory genes, Tumor Mutational Burden (TMB), tumour neoantigens, Homologous Recombination Defects (HRD), Microsatellite Instability (MSI), Single Nucleotide Variation (SNV), Copy Number Variation (CNV), tumour stemness score and enrichment analysis regarding MZT2A.
Results: MZT2A was highly expressed in most tumours and was positively or negatively correlated with the prognosis of patients with different tumours. MZT2A was also found to be negatively correlated with the immune microenvironment, immune infiltrating cells, and immune-related genes in most tumours, and was strongly correlated with TMB in 11 tumours, MSI in 10 tumours, tumour neoantigens in 5 tumours, and HRD in 12 tumours, The expression of MZT2A in 10 tumors is closely related to the presence of CNV, and MZT2A expression was significantly correlated with RNA stemness score (RNAss) in 24 tumours and DNA stemness score (DNAss) in 12 tumours. The results suggest that MZT2A can be used as a target or predictor of tumour therapy. The immunosuppressive effect of MZT2A in TME suggests that anti-MZT2A immunotherapy may be a new direction in cancer treatment.
Conclusion: MZT2A is involved in tumour development and can be used as a prognostic marker for a variety of tumours and as a target or predictor for tumour immunotherapy.
Pan-cancer; MZT2A; Tumor immunity; Prognosis biomarker; Tumor markers
ACC: Adrenocortical carcinoma; BLCA: Bladder Urothelial Carcinoma; BRCA: Breast invasive carcinoma; CESC: Cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL: Cholangiocarcinoma; COAD: Colon adenocarcinoma; COADREAD: Colon adenocarcinoma/Rectum adenocarcinoma, Esophageal carcinoma; DLBC: Lymphoid Neoplasm Diffuse Large B-cell Lymphoma; ESCA: Esophageal Carcinoma; GBM: Glioblastoma Multiforme; GBMLGG: Glioma; HNSC: Head and Neck Squamous Cell Carcinoma; KICH: Kidney Chromophobe; KIPAN: Pan-kidney cohort (KICH+KIRC+KIRP); KIRC: Kidney Renal Clear Cell Carcinoma; KIRP: Kidney, Renal, Papillary cell carcinoma; LAML: Acute Myeloid Leukemia; LGG: Brain Lower Grade Glioma; LIHC: Liver Hepatocellular Carcinoma; LUAD: Lung Adenocarcinoma; LUSC: Lung Squamous Cell Carcinoma; MESO: Mesothelioma; OV: Ovarian serous cystadenocarcinoma; PAAD: Pancreatic Adenocarcinoma; PCPG: Pheochromocytoma and Paraganglioma; PRAD: Prostate Adenocarcinoma; READ: Rectum Adenocarcinoma; SARC: Sarcoma; STAD: Stomach Adenocarcinoma; SKCM: Skin Cutaneous Melanoma; STES: Stomach and Esophageal Carcinoma; TGCT: Testicular Germ Cell Tumors; THCA: Thyroid Carcinoma; THYM: Thymoma; UCEC: Uterine Corpus Endometrial Carcinoma; UCS: Uterine Carcinosarcoma; UVM: Uveal Melanoma
Cancer is an important research component in human medicine, is one of the major causes of death and has a great impact on the quality of life of patients. To date, there is still no absolute cure for cancer [1]. In recent years, more and more therapeutic measures have emerged that have a certain efficacy on the prognosis of patients, such as the current immunotherapy, especially immune checkpoint blockade therapy [2]. With the continuous updating and improvement of several public databases, it is possible to identify new targets for cancer therapy by performing pan-cancer analysis of gene expression and assessing its clinical prognosis and the relevance of relevant signalling pathways [3]. Microtubules (MT) are unbranched, hollow tubular structures composed of microtubulin protofilaments [4]. Intracellular MT is distributed in a reticular or bundle pattern and is involved in the maintenance of cell morphology, cell polarity, cell motility and cell division. Many proteins can bind to microtubules, motor proteins kinesin and dynein, and microtubule-breaking proteins, which regulate microtubule dynamics. Abnormal expression and/or activity of these proteins may be associated with a variety of cancers, such as breast cancer [5], lung cancer [6,7], liver cancer [8], gastric cancer [9], etc. The basic component of MT is tubulin, which can be divided into α-tubulin, β-tubulin and γ-tubulin. The former two account for 85%~95% of the total tubulin, while γ-tubulin accounts for less than 1% of the total tubulin, but it is essential for the executive function of microtubules, which plays an important role in the formation of microtubules, the number and location of microtubules, the determination of microtubule polarity and cell division [10]. The study found that, mutations in genes encoding γ-tubulin can cause a decrease in the number and length of cytoplasmic MT and the absence of a mitotic apparatus composed of MT, thereby affecting cell division. γ-TuRC is the initial structure of MT assembly, which is composed of γ-tubulin and some other related proteins, and exists in the form of a 25S complex in cells. Mitotic spindle tissue protein 2 (MZT2) is considered to have a variety of potential research directions in recent years. MZT2 is a small γ-tubulin ring complex protein that exists in human cells in the form of two homologues of MZT2A and MZT2B.
Mitotic spindle tissue protein 2A (MZT2A) is a centrosomal complex found by Hutchins et al. in 2010 that mediates the formation of bipolar spindles during mitosis. The complex was isolated and named MOZART1, MOZART2A and MOZART2B [11]. MZT2A is a protein in γ-TuRC with a size of about 20 kDa. Lack of MZT2A impairs the nucleation activity of MT and slows down the rate of cell proliferation.
Studies have pointed out that, MZT2A plays an important role in the new direction of human cancer diagnosis and treatment. Exploring the expression level of MZT2A in human cancers is critical for developing new therapeutic strategies. Wang H et al. found that MZT2A mRNA and MZT2A protein were highly expressed in Non-Small Cell Lung Cancer (NSCLC). The expression of MZT2A protein in NSCLC cells was significantly higher than that in normal bronchial tissues, suggesting that MZT2A expression may promote the viability and invasion of NSCLC cells.
However, research on the role of MZT2A in cancer is limited to specific types of tumors, and there is still no pan-cancer research on the role of MZT2A in multiple cancer types. Therefore, we used multiple databases such as TCGA, Cancer Cell Line Encyclopedia (CCLE), Genotype Tissue Expression (GTEx), and Human Protein Atlas (HPA) to analyze the correlation between MZT2A expression levels in different tumors and survival prognosis, immune infiltration, immune checkpoint genes, immune regulatory genes, Tumor Mutation Burden (TMB), tumor neoantigens, HRD, Microsatellite Instability (MSI), tumor stemness scores, and enrichment analysis of MZT2A. In view of this, this study aims to explore whether the expression of MZT2A in various tumor tissues is related to the occurrence, development, prognosis, immune infiltration and immunotherapy of tumors. At the same time, MZT2A is expected to become a new strategy for the development of tumor biomarkers and targeted therapy.
Data collection and processing
We downloaded a standardized pan-cancer dataset from the UCSC database: TCGA Pan-Cancer (PANCAN, N=10535, G=60499), TCGA TARGET GTEx (PANCAN, N=19131, G=60499). The expression data of MZT2A gene in each sample was extracted and the source of the sample was screened for subsequent analysis.
Differential expression
We used the expression data of MZT2A gene in each sample in TCGA Pan-Cancer and TCGA TARGET GTEx datasets, and screening data sources: Solid tissue normal, primary solid tumor, primary tumor, normal tissue, primary blood derived cancer-bone marrow, primary blood derived cancer-peripheral blood samples and performed log2 (x+0.001) transformation on each expression value to obtain the expression data of multiple cancer species (Make the data distribution more normal and the model can be better trained to fit). The difference in expression between normal and tumor samples in each tumor was calculated using R software (version 3.6.4), and unpaired Wilcoxon Rank Sum and Signed Rank Tests were used for significance analysis.
Immunohistochemistry (IHC) staining
To assess the plausibility of differential expression of MZT2A. We analysed the TCGA database for differential expression of MZT2A between four tumour tissues (LUAD, LUSC, LIHC, STAD) and normal tissues.
IHC images of MZT2A protein expression in normal and 4 tumour tissues were downloaded from The Human Protein Atlas (HPA) and compared. Data were compared between the two databases.
Survival analysis
We used the expression data of MZT2A gene in each sample in the TCGA Pan-Cancer data set. Further we screened Metastatic samples from Primary Blood Derived Cancer-Peripheral Blood (TCGA-LAML), Primary Tumor and TCGA-SKCM, in addition, we obtained a high-quality TCGA prognostic data set from the previously published TCGA prognostic study and removed samples with a follow-up time of less than 30 days. Further log2 (x+0.001) transformation was performed on each expression value. Finally, we also removed cancer species with a sample size of less than 10 in a single cancer species.
We used the coxph function of the R package surv (version 3.2-7) to build a Cox proportional hazards regression model [12], to analyze the correlation between MZT2A expression and Overall Survival (OS), Disease-Specific Survival (DSS), Disease- Free Interval (DFI) and Progression-Free Interval (PFI) in each cancer type. Logrank test was used for statistical test to obtain prognostic significance.
In order to more clearly analyze the relationship between the expression of MZT2A and the prognosis of tumor survival, we selected tumor species with significant differences and designed KMplot for single tumor prognosis. We used the R software package maxstat (Maximally selected rank statistics with several p-value approximations version: 0.7-25) to calculate the optimal cut-off value of MZT2A, and set the minimum group sample number greater than 25%, the maximum sample number group less than 75%, and finally obtained the optimal cut-off value. Based on this, the patients were divided into high and low groups. The difference of prognosis between the two groups was further analyzed by using the survfit function of survival in R software package, and the difference of prognosis between different groups was evaluated by logrank test.
Analysis of immune cell infiltration and tumor microenvironment
We used the TCGA Pan-Cancer dataset to extract the expression data of MZT2A gene in each sample and screened the samples from primary blood derived cancer-peripheral blood (TCGA-LAML), primary tumor and TCGA-SKCM of Metastatic samples and further performed log2 (x+0.001) transformation on each expression value. In addition, the gene expression profile of each tumor was extracted, and the expression profile was mapped to GeneSymbol. Further use of R software package (IOBR timer method), and R software package (ESTIMATE). B cell, T cell CD4, T cell CD8, neutrophil, macrophage, DC infiltration scores were re-evaluated for each patient in each tumor based on gene expression. We used the corr. test function of the R package psych (version 2.1.6) to calculate the Pearson's correlation coefficient between the gene and immune cell infiltration score in each tumor to determine the significantly correlated immune infiltration score.
Analysis of immune checkpoint genes and immune regulation genes
We used the TCGA TARGET GTEx dataset to extract the expression data of MZT2A gene and 150 marker genes of five types of immune pathways (chemokine (41), receptor (18), MHC (21), immunoinhibitor (24), immunostimulator (46)), as well as 60 marker genes of two types of immune checkpoint pathway genes (inhibitory (24), stimulatory (36)) pointed out in previous studies. The source of the sample was screened, primary solid tumor, primary tumor, primary blood derived cancer-bone marrow, primary blood derived cancer-peripheral blood samples. all normal samples were deleted, and then log2 (x+0.001) transformation was performed on each expression value, and the pearson correlation between MZT2A and marker genes of five types of immune pathways and two types of immune checkpoint pathway genes was calculated.
Correlation between genomic heterogeneity and gene expression
We used the expression data of MZT2A gene in each sample in the TCGA Pan-Cancer dataset. The samples of primary blood derived cancer-peripheral blood and primary tumor were screened. In addition, we also downloaded the level4 simple nucleotide variation dataset of all TCGA samples processed by MuTect2 software from GDC. The tmb function of the R software package maftools (version 2.8.05) was used to calculate the TMB (Tumor Mutation Burden) of each tumor.
MSI (Microsatellite Instability) score of each tumor obtained from previous studies; and neoantigen (immune neoantigen) data and HRD (Homologous Deficiency Recombination) data for each tumor obtained in previous studies. Then TMB, MSI, Neoantigen, HRD (homologous recombination deficiency) and gene expression data were integrated. Further, a log2 (x+0.001) transformation is performed for each expression value.
Correlation between gene expression and mutation
We used the expression data of MZT2A gene in each sample from TCGA pan-cancer dataset, and we also downloaded the level4 simple nucleotide variation dataset of all TCGA samples processed by MuTect2 software from GDC. We also downloaded the simple nucleotide variation dataset of all TCGA samples processed by MuTect2 software from GDC, screened samples from primary blood derived cancer-peripheral blood, primary tumor, and we integrated the mutation data and gene expression data of the samples; in addition, we downloaded the nucleotide variation dataset from GDC, we downloaded the copy number variation dataset of all TCGA samples processed by GISTIC software at the level4 gene level [13], and we integrated the copy number data and gene expression data of the samples expression data. Further, log2 (x+0.001) transformation was performed for each expression value.
Correlation between tumor stemness and gene expression
We extracted the expression data of MZT2A gene in each sample using TCGA Pan-Cancer dataset and screened the samples from primary blood derived cancer-peripheral blood, primary tumor, the DNAss tumor stemness score calculated by methylation characteristics and the RNAss tumor stemness score calculated by mRNA characteristics were obtained from each tumor obtained in the previous study. The stemness index and gene expression data of the sample were integrated, and the log2 (x+0.001) transformation was further performed on each expression value.
Gene set enrichment analysis
To reflect the biological potential of MZT2A and to explore the expression of its signaling pathways and immune functions in tumors, we enriched the genes in the GSEA predefined gene set as references for evaluating the aberrantly expressed MZT2A enriched in the KEGG and HALLMARK sets, respectively [14]. Finally, the net enrichment score (NES) was calculated to find the significantly enriched pathways, with False Discovery Rate (FDR) <0.05 as the critical standard.
Statistics
We used unpaired Wilcoxon Rank Sum and Signed Rank Tests for significance of differences, and Kaplan-Meier curves, log-rank tests, and Cox proportional risk regression models were used for all survival analyses in this study. Correlation analysis between two variables was performed using the Spearman test; P<0.05 was considered significant. All statistical analyses were processed by R software (version 3.6.4).
Expression of MZT2A in various normal and cancer tissues
We obtained the differences in gene expression between tumors and normal tissues in each tumor sample from the TCGA database. As shown in Figure 1A, MZT2A differential expression was statistically significant in 20 of 26 tumor samples. Considering that there are few normal samples in TCGA, we integrated the data of normal tissues in GTEx database and the data of TCGA tumor tissues to analyze the expression differences of 34 tumors, as shown in Figure 1B. Among them, there were significant statistical differences in the expression of MZT2A between 25 tumors and normal tissues. In the Figure 1, *represents P<0.05, **represents P<0.01, ***represents P<0.001.
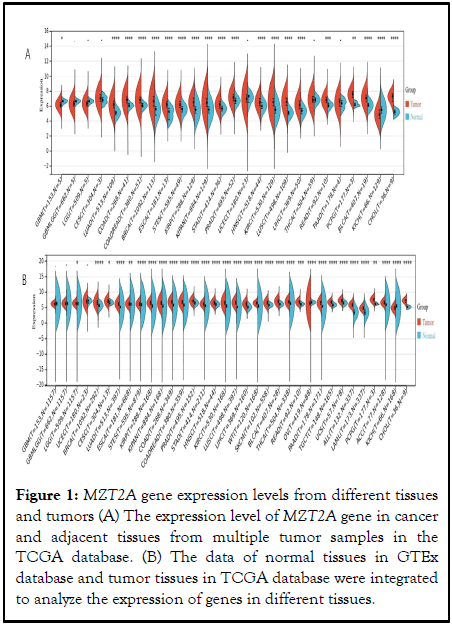
Figure 1: MZT2A gene expression levels from different tissues and tumors (A) The expression level of MZT2A gene in cancer and adjacent tissues from multiple tumor samples in the TCGA database. (B) The data of normal tissues in GTEx database and tumor tissues in TCGA database were integrated to analyze the expression of genes in different tissues.
Immunohistochemistry (IHC) staining
In order to verify the TCGA database about the expression level of MZT2A in different tissues and tumors, we analyzed the IHC results provided in the HPA database and compared them with the expression data of MZT2A in the TCGA database, such as Figures 2A-D. The results show that the analysis results of the two databases are consistent. Normal lung, liver and stomach tissues have low and moderate MZT2A IHC staining, while tumor tissues have moderate and strong staining. The differential expression data of MZT2A in normal tissues and tumor tissues analyzed by TCGA database were basically the same [15].
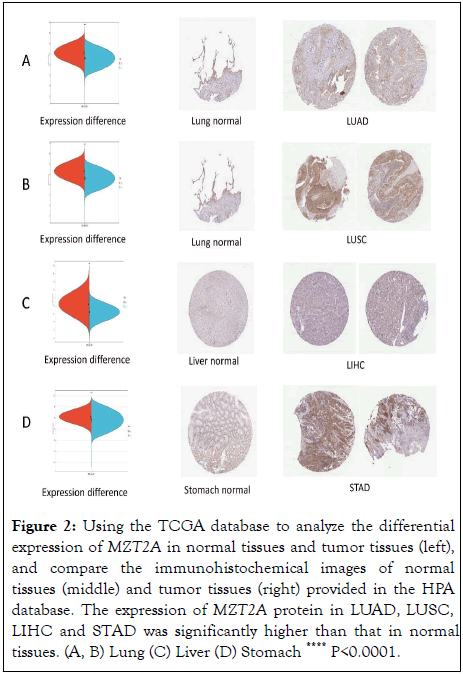
Figure 2: Using the TCGA database to analyze the differential expression of MZT2A in normal tissues and tumor tissues (left), and compare the immunohistochemical images of normal tissues (middle) and tumor tissues (right) provided in the HPA database. The expression of MZT2A protein in LUAD, LUSC, LIHC and STAD was significantly higher than that in normal tissues. (A, B) Lung (C) Liver (D) Stomach ****P<0.0001.
Correlation between MZT2A expression level and tumor prognosis
In order to study the relationship between the expression level of MZT2A and the prognosis of patients, survival analysis was performed in each cancer, including OS, DSS, DFI and PFI. COX proportional hazard model showed that there were 9 tumor types with poor prognosis: LUAD (p=3.9e-4), LAML (p=0.02), KIRP (p=2.1e-4), KIPAN (p=4.4e-7), KIRC (p=0.04 ), LIHC (p=0.04), SKCM-M (p=0.02), SKCM (p=0.02 ), KICH (p=0.02). Low expression in GBMLGG (p=3.4e-3), LGG (p=0.02), BRCA (p=0.02), OV (p=0.02) had poor prognosis, (Figure 3A). Four cancer types (P<0.01) were selected from the above cancers, namely LUAD, KIRP, KIPAN, and GBMLGG. The Kaplan-Meier curve results showed that in LUAD (P=3.0e-4), KIRP (P=1.2e-5), KIPAN (P=1.3e-7), patients with high expression levels of MZT2A had shorter survival time. In GBMLGG (P=1.4e-3), the low expression level of MZT2A had shorter survival time (Figures 3B-E).
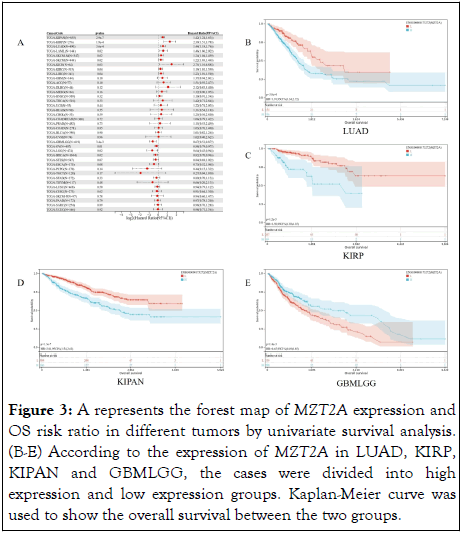
Figure 3: A represents the forest map of MZT2A expression and OS risk ratio in different tumors by univariate survival analysis. (B-E) According to the expression of MZT2A in LUAD, KIRP, KIPAN and GBMLGG, the cases were divided into high expression and low expression groups. Kaplan-Meier curve was used to show the overall survival between the two groups.
Considering the presence of non-tumor deaths during tumor sample collection, OS may be affected. Therefore, we used Cox survival analysis to evaluate the correlation between MZT2A expression level and DSS (disease-specific survival) in different tumors. The results showed that high expression in 7 tumor types LUAD (p=7.9e-3), KIRP (p=3.6e-8), KIPAN (p=4.5e-10), KIRC (p=3.7e-4), SKCM-M (p=0.01), SKCM (p=0.02), KICH (p=0.01) had poor prognosis, and low expression in 3 tumor types GBMLGG (p=3.5e-4), LGG (p=0.01), OV (p=6.5e-3) had poor prognosis (Figure 4A). Five cancer types were selected from the above cancers (p<0.01), namely KIPAN, KIRC, KIRP, LUAD, and GBMLGG. The Kaplan-Meier curve results showed that in KIPAN (p=2.2e-11), KIRC (p=3.4e-6), KIRP (p=1.1e-9), LUAD (p=5.5e-4), patients with high expression levels of MZT2A had shorter survival time. In LGG (p=8.5e-5), low expression of MZT2A had a worse prognosis (Figures 4B-F).
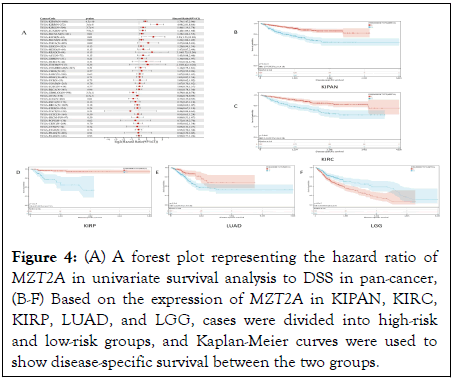
Figure 4: (A) A forest plot representing the hazard ratio of MZT2A in univariate survival analysis to DSS in pan-cancer, (B-F) Based on the expression of MZT2A in KIPAN, KIRC, KIRP, LUAD, and LGG, cases were divided into high-risk and low-risk groups, and Kaplan-Meier curves were used to show disease-specific survival between the two groups.
The correlation between MZT2A expression and DFI was analyzed. Finally, it was observed that high expression in five tumor types KIRP (p=0.02), KIPAN (p=8.4e-3), PRAD (p=0.04), LIHC (p=0.03), ACC (p= 0.04) had poor prognosis, and low expression in one tumor type (PCPG (p=6.9e-3)) had poor prognosis, (Figure 5A). Two types of cancer were selected from the above cancers (P<0.01), which were KIPAN and PCPG, respectively. Kaplan-Meier curve results showed that in KIPAN (P=3.7e-5), patients with high MZT2A expression had shorter survival time. In PCPG (P=0.02), low expression of MZT2A had a worse prognosis (Figures 5B-C).
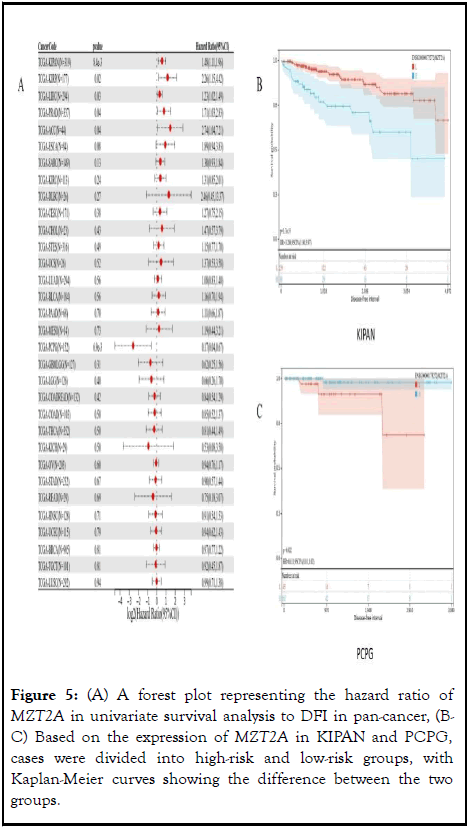
Figure 5: (A) A forest plot representing the hazard ratio of MZT2A in univariate survival analysis to DFI in pan-cancer, (BC) Based on the expression of MZT2A in KIPAN and PCPG, cases were divided into high-risk and low-risk groups, with Kaplan-Meier curves showing the difference between the two groups.
Similarly analyzing the correlation between MZT2A expression and PFI, forest-like plots showed that high expression was observed in 5 tumor types (KIRP (N=273, p=7.9e-6, KIPAN (p=7.0e-14), KIRC (p=1.5e-7), LIHC (p=0.01), TCGA-ACC (p=7.1e-4)) with poor prognosis, low expression in 3 tumour types GBMLGG (p=1.1e-5), LGG (p=3.7e-3), OV (p=9.9e-3) had poor prognosis (Figure 6A ). 5 cancer types were selected from the above cancers (p<0.01), namely ACC, KIPAN, KIRC, KIRP, GBMLGG, LGG and Kaplan-Meier was generated. The results of the curves showed that patients with high expression levels of MZT2A had shorter survival times in ACC (p=9.6e-4), KIPAN (p=1.3e-13), KIRC (p=5.9e-9),KIRP (p=2.0e-8). In GBMLGG (p=1.0e-4), LGG (p=4.5e-3), MZT2A low expression had a worse prognosis (Figures 6B-G).
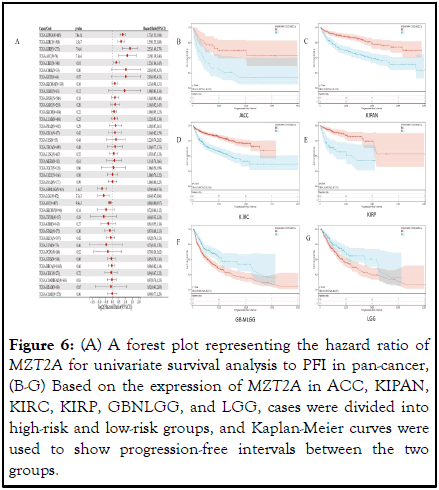
Figure 6: (A) A forest plot representing the hazard ratio of MZT2A for univariate survival analysis to PFI in pan-cancer, (B-G) Based on the expression of MZT2A in ACC, KIPAN, KIRC, KIRP, GBNLGG, and LGG, cases were divided into high-risk and low-risk groups, and Kaplan-Meier curves were used to show progression-free intervals between the two groups.
Analysis of immune cell infiltration and tumor microenvironment
The expression data of MZT2A in different tumors were extracted from the TCGA database, and the B cell, T cell CD4, T cell CD8, neutrophil, macrophage, and DC infiltration scores of each patient in each tumor were evaluated according to gene expression, (Figure 7). The results showed that the expression of MZT2A was significantly correlated with immune infiltration in 32 tumors. Except LIHC and KICH, the expression of MZT2A was negatively correlated with immune infiltrating cells in other tumors.
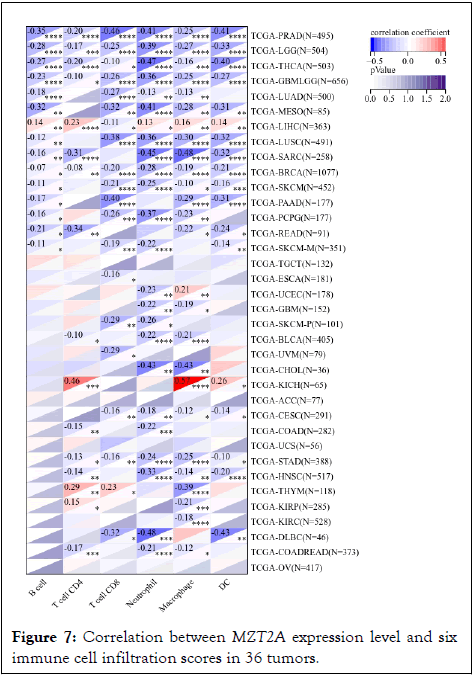
Figure 7: Correlation between MZT2A expression level and six immune cell infiltration scores in 36 tumors.
Tumor microenvironment is an indispensable factor in the field of tumor research, which plays a vital role in the development of tumor [16]. Therefore, it is of great significance to analyze the relationship between TME and MZT2A expression in pan-cancer research. After analyzing the immune infiltration scores of 44 tumor samples, it was observed that the expression of MZT2A in 32 cancer species was significantly correlated with stromal score, of which 30 were significantly negatively correlated and 2 were significantly positively correlated. Similarly, MZT2A expression was observed to be significantly correlated with immune scores in 27 cancer species, of which 24 were significantly negatively correlated and 3 were significantly positively correlated. The three tumors with the highest negative correlation coefficient and the tumors with positive correlation were listed in the following Figure 8. We found that the correlation coefficient was the highest in GBMLGG, LUSC, SARC, and THCA. The expression of MZT2A was significantly negatively correlated with immune score and stromal score, while KIPAN, KIRC, and THYM were the few tumors with positive correlation (Figure 8).
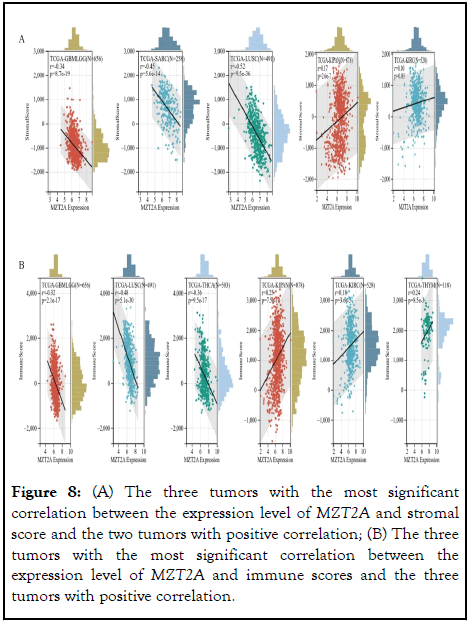
Figure 8: (A) The three tumors with the most significant correlation between the expression level of MZT2A and stromal score and the two tumors with positive correlation; (B) The three tumors with the most significant correlation between the expression level of MZT2A and immune scores and the three tumors with positive correlation.
Analysis of immune checkpoint genes and immune regulation genes
In addition, to further evaluate the role of MZT2A in tumor immunity, we also analyzed the co-expression of MZT2A and 210 genes in different tumors, including 150 marker genes of 5 types of immune pathways (chemokine (41), receptor (18), MHC (21), immunoinhibitor (24), immunostimulator (46)) and 60 genes of 2 types of immune checkpoint pathways (inhibitory (24), stimulatory (36)) (Figures 9A,B). The results showed that MZT2A was negatively correlated with most of these genes in different tumors. Similar to the correlation of immune infiltrating cells, this may also be a special role of MZT2A in the field of tumor immunity, with TME specificity, providing a new target for tumor immunotherapy.
In summary, MZT2A has the potential to become a target for cancer immunotherapy. MZT2A has an immunosuppressive effect in TME, suggesting that anti-MZT2A immunotherapy may be a new direction in cancer treatment.
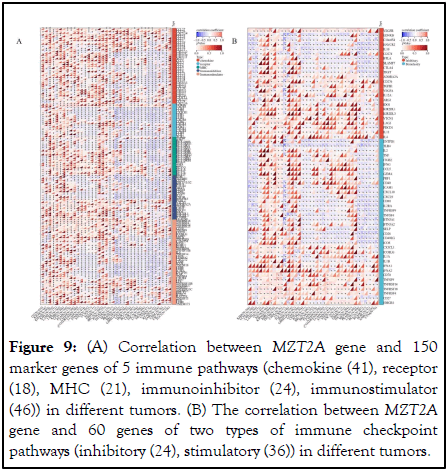
Figure 9: (A) Correlation between MZT2A gene and 150 marker genes of 5 immune pathways (chemokine (41), receptor (18), MHC (21), immunoinhibitor (24), immunostimulator (46)) in different tumors. (B) The correlation between MZT2A gene and 60 genes of two types of immune checkpoint pathways (inhibitory (24), stimulatory (36)) in different tumors.
Correlation between genomic heterogeneity and gene expression
At present, TMB and MSI are considered as biomarkers and immunotherapy predictors for evaluating tumor prognosis. In view of this, this study analyzed the correlation between MZT2A expression and TMB and MSI in different tumors. The results showed that the expression of MZT2A was significantly correlated with TMB in 10 tumors. Among them, there was a significant positive correlation in 8 tumors, such as LUAD, STES, KIPAN, STAD, UCEC, HNSC, LIHC, THCA, and a significant negative correlation in 2 tumors, such as LAML, CHOL, (P<0.05), (Figure 10A). It was observed that the expression of MZT2A was significantly correlated with MSI in 11 tumors, which was significantly positively correlated in 10 tumors, such as GBM, GBMLGG, LUAD, STES, STAD, PRAD, HNSC, LUSC, LIHC, DLBC, and significantly negatively correlated in 1 tumor, such as KIPAN (Figure 10B, P<0.05). Tumor neoantigen is a new tumor immunotherapy method. In order to further understand the value of MZT2A in the field of immunotherapy, we analyzed the correlation between MZT2A expression and NEO in different tumors. The results showed that the expression of MZT2A was significantly correlated with NEO in five tumors. Among them, there was a significant positive correlation in three tumors, such as LUAD, UCEC and HNSC, and a significant negative correlation in two tumors, such as SARC and CHOL (Figure 10C, p<0.05). Homologous Recombination Defect (HRD) leads to impaired repair of double-strand breaks, which is one of the driving factors of tumorigenesis. Studies have pointed out that [17], HRD status is a key indicator of treatment options and prognosis of various tumors. Therefore, we analyzed the correlation between MZT2A expression and HRD in different tumors, and observed a significant correlation in 12 tumors, of which a significant positive correlation in 10 tumors, such as GBM, LUAD, KIRP, KIPAN, HNSC, KIRC, LUSC, LIHC, MESO, KICH, and a significant negative correlation in 2 tumors, such as BRCA, PRAD (p<0.05, as in Figure 10D).
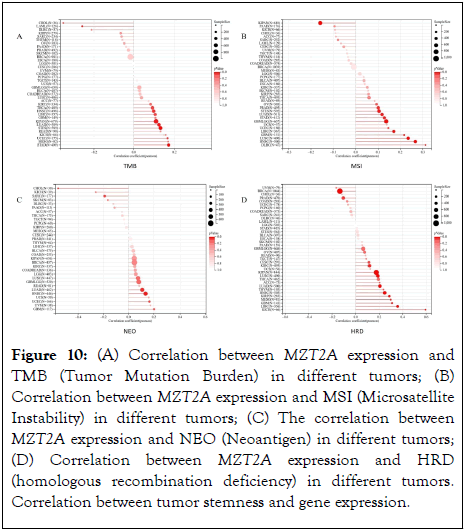
Figure 10: (A) Correlation between MZT2A expression and TMB (Tumor Mutation Burden) in different tumors; (B) Correlation between MZT2A expression and MSI (Microsatellite Instability) in different tumors; (C) The correlation between MZT2A expression and NEO (Neoantigen) in different tumors; (D) Correlation between MZT2A expression and HRD (homologous recombination deficiency) in different tumors. Correlation between tumor stemness and gene expression.
Correlation between gene expression and mutation
Single Nucleotide Variation (SNV) refers to DNA sequence variation caused by single nucleotide changes at the genomic level. Copy Number Variation (CNV) generally refers to an increase or decrease in the copy number of a large segment of the genome of more than 1 kb in length, mainly in the form of deletions and duplications at the submicroscopic level. As an important biological indicator of disease, mutations and changes in genomic deletions or amplifications have become an important part of current tumor research. In order to comprehensively understand the pathogenic role of MZT2A in different tumors, we analyzed the correlation between MZT2A expression and SNV and CNV in different tumors. Finally, we observed data on the correlation between MZT2A expression and SNV in three tumors, but none of them were significantly different (P<0.05, as in Figure 11A); in addition, we observed significant differences in the correlation between MZT2A expression and CNV in 10 tumors such as BRCA, ESCA, STES, SARC, STAD, PRAD, HNSC, LUSC LIHC, and OV (P>0.05, as in Figure 11B).
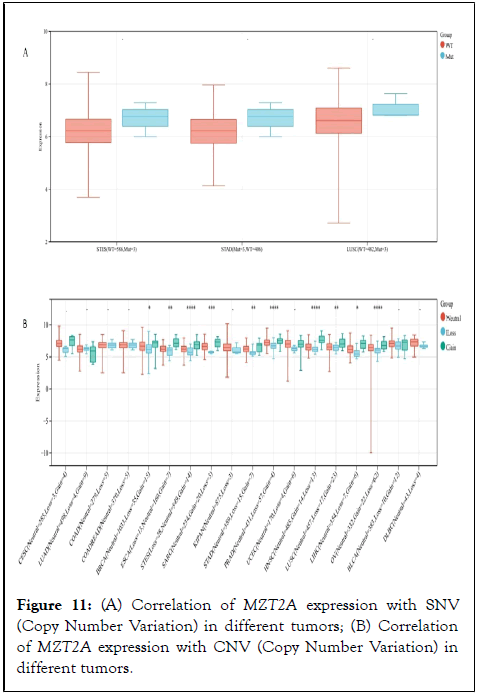
Figure 11: (A) Correlation of MZT2A expression with SNV (Copy Number Variation) in different tumors; (B) Correlation of MZT2A expression with CNV (Copy Number Variation) in different tumors.
Correlation between tumor stemness and gene expression
Increased expression of stemness-related biomarkers in tumor cells is highly correlated with drug resistance during tumor treatment, tumor recurrence, and tumor proliferation. Therefore, we evaluated the correlation of MZT2A expression with RNA stemness scores (RNAss) and DNA stemness scores (DNAss) in different tumors. The results showed that the expression of MZT2A was significantly correlated with RNA stemness scores (RNAss) in 24 tumors. Among them, there was a significant positive correlation in 20 tumors, such as GBMLGG, LGG, CESC, LUAD, COAD, COADREAD, ESCA, STES, SARC, STAD, UCEC, HNSC, LUSC, THYM, LIHC, MESO, READ, SKCM, BLCA, DLBC, and a significant negative correlation in 4 tumors, such as BRCA, KIPAN, KIRC and TGCT (Figure 12A). We observed that the expression of MZT2A was significantly correlated with DNA stemness scores (DNAss) in 12 tumors. Among them, there was a significant positive correlation in 4 tumors, such as HNSC, LUSC, PAAD, TGCT, and a significant negative correlation in 8 tumors, such as CESC, KIPAN, PRAD, THYM, LIHC, THCA, READ, CHOL (Figure 12B).
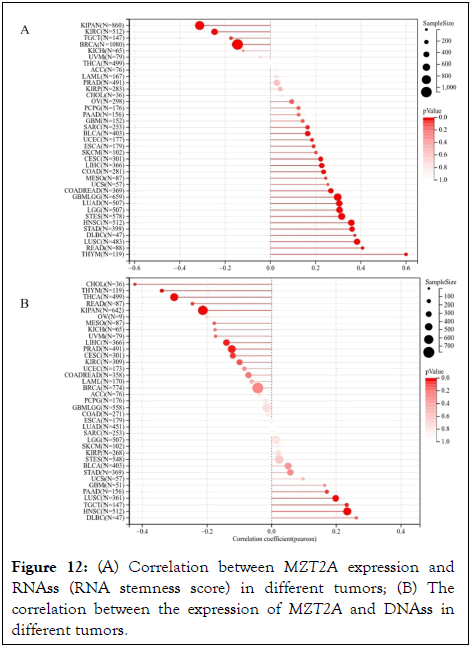
Figure 12: (A) Correlation between MZT2A expression and RNAss (RNA stemness score) in different tumors; (B) The correlation between the expression of MZT2A and DNAss in different tumors.
Gene annotation and pathway enrichment analysis
To explore the impact of the signaling pathway and immune function of MZT2A in tumors, we divided the samples into high and low expression groups based on gene expression, and enriched genes in the GSEA predefined gene set were used as an assessment of the enrichment of different MZT2A expression groups in the KEGG and HALLMARK pathways. We considered enrichment with an absolute value of NES (Normalized Enrichment Score) greater than 1, NOM (nominal) P<0.05, and FDR (false discovery rate) q-value<0.25 to be statistically significant. Visualizing the top three most significant pathways as follows, we found that KEGG, HALLMARK enrichment in the high expression group in A and C showed that overexpression of MZT2A was associated with pyrimidine metabolism (NES=-2.2, NOM P<0.05, FDR=0.0026), DNA repair (NES=-2.2, NOM P<0.05, FDR=0.0031), oxidative phosphorylation (NES=-2.1, NOM) P<0.05, FDR=0.0051), and MYC targets v2 (NES=-2.1, NOM P<0.05, FDR=0.0044) were correlated, as detailed in Figure 11. In addition, hypermetabolism of MZT2A was associated with neurological pathologies such as Huntington's disease (NES=-2.2, NOM P<0.05, FDR=0.0031) and Alzheimer's disease (NES=-2.1. NOM P<0.05, FDR=0.0026) (Figures 13 A-D).
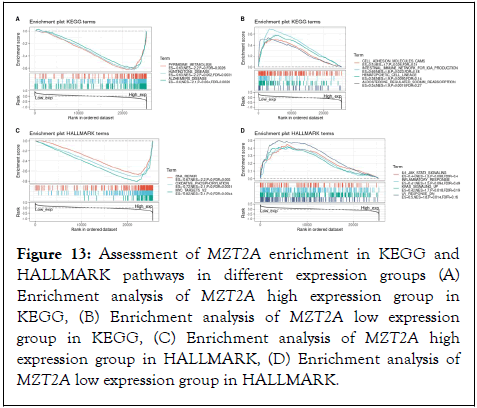
Figure 13: Assessment of MZT2A enrichment in KEGG and HALLMARK pathways in different expression groups (A) Enrichment analysis of MZT2A high expression group in KEGG, (B) Enrichment analysis of MZT2A low expression group in KEGG, (C) Enrichment analysis of MZT2A high expression group in HALLMARK, (D) Enrichment analysis of MZT2A low expression group in HALLMARK.
Pan-cancer analysis can distinguish the similarities and differences of gene expression in different cancers, and help to improve human understanding of the prevention of tumor diseases and new tumor markers. A number of pan-cancer studies have concluded that there is a certain correlation between tumor development and gene expression and gene mutation. Monitoring gene expression is helpful to evaluate disease prognosis and develop new directions for diagnosis and treatment. MZT2A mediates cell septum signal transduction by aggregation and depolymerization at the centrosome MT [18]. The lack of MZT2A will weaken the nucleation activity of MT and slow down the rate of cell proliferation, and participate in the regulation of tumor cell cycle and cell proliferation. Wang H et al. found that high expression of MZT2A can promote the invasion of NSCLC tumor cells, and its overexpression level is associated with NSCLC progression and poor prognosis. However, there are few reports on the expression of MZT2A in different tumors and its relationship with disease prognosis and tumor development. In view of this, this study explores.
MZT2 is a small gamma-tubulin circular complex protein that exists in human cells in the form of two homologues, MZT2A and MZT2B, which are approximately 96 % identical. Previous studies have pointed out that, MZT2B was found to be highly expressed in gastric antrum and gastric body of female patients over 50 years old with Gastric Cancer (GC). Such patients are prone to distant metastasis, so that the clinical stage and prognosis are poor. As an important binding protein in mitotic spindle tissue, MZT2B has been found to be involved in the occurrence and development of gastric cancer. The increased expression of MZT2B is closely related to the activation of MYC gene, which leads to great changes in extracellular matrix and cell communication pathways, and is related to risk factors such as tumor progression, intestinal deformation and Helicobacter pylori infection. The analysis of MZT2 in breast cancer only found one report that the MYC gene regulates MZT2B expression in breast cancer tissue gene expression data. MZT2 may affect the occurrence and development of tumors, but the specific mechanism is still controversial. With the gradual discovery of the role of MZT2B, MZT2A, which has the core subunit of γTuRC, has not yet started systematic bioinformatics research. Prior to this project, only Wang H et al. found that MZT2A messenger RNA and MZT2A protein levels were highly expressed in Non-Small Cell Lung Cancer (NSCLC) ; the expression level of MZT2A protein in NSCLC cells was significantly higher than that in normal bronchial tissues, suggesting that MZT2A expression may promote the viability and invasion of NSCLC cells. However, whether the expression level of MZT2A in different tumors is related to the survival prognosis, immune infiltration, immune checkpoint genes, immune regulation genes, tumor mutation load (TMB), tumor neoantigen, HRD, Microsatellite Instability (MSI), tumor stemness score and the enrichment analysis of MZT2A remains to be studied. In summary, MZT2A expression may play a key role in the development of different tumors and may affect the prognosis of patients. In this study, by comparing the differential expression of MZT2A between different tumors and normal tissues, it was found that MZT2A was highly expressed in various tumor tissues. The results of survival analysis showed that the expression of MZT2A was positively correlated with the prognosis of most tumor patients, especially in LUAD, KIPAN, KIRC, KIRP, GBMLGG and LGG, suggesting that the high expression of MZT2A was closely related to the decrease of survival time of these tumor patients. The expression of MZT2A was negatively correlated with the prognosis of GBMLGG and LGG, suggesting that the high expression of MZT2A was closely related to the prolonged survival of these two cancer patients. At the same time, MZT2A is expected to become a new target for future cancer treatment.
Tumor immunity and TME have always been the focus of tumor research. Immune cells are important substrates in TME, including B cells, CD4 T cells, CD8 T cells, neutrophils, macrophages and dendritic cells. A number of studies have pointed out that, immune cells are one of the key factors in a variety of tumor development, can antagonize or promote tumor progression. The results of this study showed that the expression of MZT2A in various tumors was negatively correlated with six immune cells, especially in COAD, LGG and LUAD. At the same time, we also analyzed the correlation between MZT2A expression and immune score, matrix score in a variety of tumors. The results showed that MZT2A expression was significantly correlated with matrix score and immune score in most tumors. It indicates that MZT2A may inhibit or promote tumor progression by regulating immune cells. This provides a new idea for us to explore the specific mechanism of MZT2A in tumor microenvironment. In addition, this study also revealed that MZT2A was co-expressed with immune pathway marker genes and immune checkpoint genes. These results suggest that the expression of MZT2A is closely related to the immune infiltration of tumor cells and affects the prognosis of patients, providing a new target for immunosuppressive agents.
Tumor Mutational Burden (TMB) is usually measured by the number of somatic mutations in the average 1Mb base in the coding region of the tumor cell genome. TMB can indirectly reflect the ability and extent of tumor to produce new antigens and predict the efficacy of immunotherapy for a variety of tumors [19]. Microsatellites are short tandem repeats distributed throughout the human genome, with single nucleotide, double nucleotide or high nucleotide repeats, repeat number 10-50 times. Compared with normal cells, microsatellites in tumor cells become microsatellite instability due to the insertion or deletion of repeat units leading to changes in microsatellite length. MSI is an important biomarker of Immune Checkpoint Inhibitors (ICI). It is also an independent predictor of clinical features and prognosis of colorectal cancer. The results of this study showed that MZT2A expression was closely related to TMB of 12 tumor types and MSI of 11 tumors, which may indicate that MZT2A expression may affect TMB and MSI of tumors. This means that MZT2A may be a new marker for evaluating the immune effect of tumors and provide a reference for tumor immunotherapy. Neoantigens are patient-specific tumor antigens caused by mutations in the process of tumorigenesis and are very valuable targets for tumor immunotherapy. Therefore, evaluating the correlation between MZT2A expression and tumor neoantigens is helpful to evaluate the value of genes in tumor immunotherapy. Studies have found that MZT2A expression is closely related to five types of tumor neoantigens. HRD status is an important indicator to evaluate the treatment and prognosis of various tumors. Studies have shown that HRD can predict the therapeutic effect of neoadjuvant immunotherapy in NSCLC patients; it is also an important factor leading to some cancers, such as ovarian cancer; it is also highly correlated with the sensitivity of platinum chemotherapy drugs and PARP inhibitors. Therefore, it is important to evaluate the correlation between MZT2A expression and HRD status in pan-cancer. The results of this study showed that MZT2A expression was closely related to HRD in 12 tumor types, once again revealing that MZT2A may be a target or predictor for pan-cancer diagnosis and treatment.
Cancer stem cells have the characteristics of self-renewal and can produce heterogeneous tumor cells, which play an important role in tumor survival, metastasis, proliferation and recurrence [20]. RNAss is the stemness index calculated from tumor expression data, and DNAss is the stemness index calculated from methylation data. The stemness index closer to 1, the lower the degree of cell differentiation, stem cell characteristics stronger. The results of this study showed that the expression of MZT2A was significantly correlated with RNA stemness scores (RNAss) in 24 tumors, among which there was a significant positive correlation in 20 tumors, such as GBMLGG, LGG, CESC, LUAD, etc. ; the expression of MZT2A was significantly correlated with the DNA stemness score ( DNAss) of 12 tumors, among which there was a significant positive correlation in 4 tumors, such as HNSC, LUSC, PAAD, TGCT, indicating that the higher expression of MZT2A in these tumors is conducive to promoting tumor proliferation and metastasis. Negative correlation with dryness score is the opposite. Correlation can also be used as a predictor of efficacy of immune checkpoint inhibitors.
In summary, for the first time, the analysis of MZT2A in pan-cancer showed that the gene was differentially expressed between tumors and normal tissues and was associated with the prognosis of patients with various tumors. Through the analysis of various data, MZT2A can be used as an independent prognostic factor for many tumors. Different levels of expression may lead to different prognostic results. At the same time, it was found that its correlation in the field of tumor immunity has obvious specificity, for example, it is negatively correlated with most of the immune microenvironment, immune infiltrating cells, immune-related genes, and the results are consistent. All indicate that MZT2A has the potential to become a target for cancer immunotherapy. Moreover, MZT2A is associated with TMB, MSI, tumor neoantigen, HRD and CNV in a variety of tumors, once again confirming that MZT2A can be used as a target or predictor for tumor treatment. In addition, the expression of MZT2A was significantly correlated with tumor RNAss and DNAss. These findings suggest that MZT2A may be involved in tumorigenesis and development, and provide a reference for future precision treatment.
The datasets generated and/or analysed during the current study are available in the UCSC database. Immunohistochemistry images of MZT2A protein expression were downloaded from the Human Protein Atlas (HPA). All the datasets were open access datasets.
The authors declare that this study does not involve any institutional or personal conflict of interest.
ZS conceived this study. ZS and LR were responsible for sorting out and writing articles. WW and YM were responsible for collecting and sorting out data. LD, LJ, LS and ZQ collected literature and provided ideas. LR was responsible for reviewing manuscripts. All authors read and approved the final manuscripts.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Zhan S, Wang W, Yan M, Li D, Liu J, Fan X, et al. (2025) Correlation of MZT2A Expression with Immunity and Prognosis in Pan-Cancer Analysis. Immunome Res. 21:292.
Received: 27-Feb-2023, Manuscript No. IMR-23-21963; Editor assigned: 02-Mar-2023, Pre QC No. IMR-23-21963 (PQ); Reviewed: 17-Mar-2023, QC No. IMR-23-21963; Revised: 02-Jan-2025, Manuscript No. IMR-23-21963 (R); Published: 09-Jan-2025 , DOI: 10.35248/1745-7580.25.21.292
Copyright: © 2025 Zhan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.