Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Review Article - (2023)Volume 14, Issue 4
Disorders of the cornea can be interpreted to a large extent based on the structural uniqueness of its layers. Here, we attempt to correlate the corneal anatomy with some of its pathology. This would aid better teaching as well as understanding of common corneal diseases.
Common and unique corneal diseases affecting the corneal epithelium, Bowman’s layer, stroma, Descemet’s membrane and endothelium, seen routinely in our tertiary care corneal practice have been enlisted. Disorders altering the structural characteristics of the cornea, such as its front and back curvature, thickness, neurovascular supply etc., have also been included. The relevant anatomical characteristics of these structures have been referenced from peer-reviewed literature and standard textbooks for correlation.
An attempt has been made to correlate the disease pathology of important corneal conditions with the anatomic characteristics of its layers. Embryological and structural connotations of corneal disease were also studied. The results depict the relevant applied clinical anatomy of corneal diseases which has not been compiled before in literature.
Corneal layers; Applied anatomy; Bowman’s membrane; Endothelium
The cornea is the anterior refractive unit of the human eye. The distinctive lamellar arrangement of collagen fibers and lack of vascularity confer transparency and immune privilege to the cornea, which is unlike most other body tissues. The cornea is bathed by the pre-corneal tear film and is five-layered. Disorders of the cornea can be interpreted to a large extent based on the structural uniqueness of these layers. Here, we attempt to correlate the corneal anatomy with some of its pathology. This would aid better teaching as well as understanding of common corneal diseases.
Corneal embryology and its applied anatomy
The corneal epithelium is derived from the surface ectoderm and is the first layer of the cornea to develop. The corneal stroma, including the endothelium, is derived from neural crest cells [1]. The Descemet’s membrane is the only layer which completes its development postnatally. The Bowman’s membrane and the endothelium do not have much regenerative potential (Table 1).
| Microscopic layer | Embryological layer | Formation at |
|---|---|---|
| Epithelium | Surface ectoderm | 5-6 weeks of gestation |
| BM | Neural crest cells | 7 weeks of gestation |
| Stroma | Neural crest cells | 7 weeks of gestation |
| DM | Mesenchymal cells from neural crest cells | Pre+postnatal |
| Endothelium | Neural crest cells | 8 weeks of gestation |
Note: BM: Bowman's Membrane; DM: Descemet's Membrane
Table 1: Corneal embryology showing the regenerative potential.
Structures derived from the neural crest cells lack regenerative capacity, typical examples being endothelium and Bowman’s membrane. Bowman’s membrane has the least renewal potential, followed by the endothelium. Hence, injury to these results in the formation of Bowman’s scar and endothelial decompensation, respectively.
Layers of cornea and their clinical importance
It includes epithelium, Bowman’s membrane, stroma, Dua’s layer, Descemet’s membrane and endothelium.
Epithelium: The corneal epithelium is stratified squamous non- keratinized in nature and 50-90 micron thick. It has three types of cells from below upwards. They include the following:
• Basal cells or the germinative layer, having a palisade-like arrangement. These are connected with each other and to the wing cells by desmosomes and to the basal lamina by Hemi-desmosomes (HD). Peripheral basal cells have germinative potential.
• Wing or umbrella cells that is polyhedral in shape.
• Surface cells with microvilli, comprising 2-3 layers. The cells are connected to each other by zonulae occludentes.
Thoft and Friend proposed, on the basis of experimental evidence that there was both a limbal basal and a corneal basal epithelial source for corneal epithelial cells (the XYZ hypothesis) [2].
Defective HD attachments lead to instability of the overlying corneal epithelium and cause Recurrent Corneal Erosion Syndrome (RCES) as shown in Figure 1A. The newly formed epithelium also sheds off easily due of the wind-shield wiper effect of the eyelids, before HD attachments get strengthened. In inherited storage disorders, deposits can be formed along the HD, forming physical bumps, adding to the epithelial instability. Diabetic corneal neuropathy can also induce the development of persistent corneal epithelial defects [3].
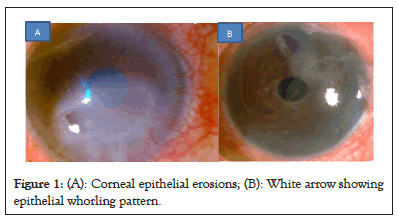
Figure 1: (A): Corneal epithelial erosions; (B): White arrow showing epithelial whorling pattern.
In cases of surface inflammation and mild limbal cell deficiency, the centripetal migration of epithelial cells slows down. Stagnation of tears on these epithelial cells with iron deposits laden, results in characteristic epithelial whorling. In inherited storage disorders, intra-cellular inclusion of lipid in the basal cells and of lipid and mucopolysaccharide in the wing and superficial cells also cause whorling pattern as shown in Figures 1A and 1B [4].
Bowman’s membrane: The Bowman’s Membrane (BM) or layer is modified anterior corneal stroma, also called the anterior limiting lamina. It is acellular in nature and 8-14 microns thick. Collagen fibrils present in the Bowman’s membrane are smaller (24 nm to 27 nm) than in the substantia propria. The lamina densa and unmyelinated nerve fibers traverse through the BM. Intermingling of fibers with the anterior stroma confers strength to the cornea. It is non-regenerative in nature. Hence, after insult to the Bowman’s membrane scarring takes place [5].
In progressive pterygium, degeneration of Bowman’s layer occurs at the head of the pterygium resulting in Bowman’s scar as shown in Figure 2. When a patient undergoes extensive Photorefractive Keratectomy (PRK), laser ablation can destroy the Bowman’s layer. The scar formed then persists causing unexpected refractive outcomes in patients. Attempts of Bowman’s layer transplant have been reported in literature in such PRK patients to remove the scar and replace it with healthy BM tissue [6]. The same principle has later been applied in cases of advanced keratoconus in which the acellular, transparent healthy Bowman’s layer is transplanted to confer strength to the ectatic cornea (Figure 2) [7].
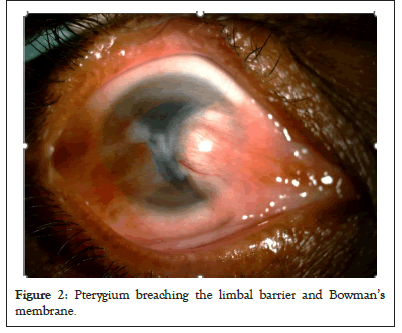
Figure 2: Pterygium breaching the limbal barrier and Bowman’s membrane.
Stroma: This is the middle, thickest layer of the cornea, measuring around 500 microns in central thickness. Collagen fibers present in the stroma are arranged in a lamellar pattern, parallel to each other centrally and circular peripherally near the limbus. Precise arrangement and inter fibrillar separation give the stroma transparency. The stroma is avascular and contains keratocytes, lymphocytes and macrophages.
The limbal stem cell barrier prevents superficial vascularization into the cornea, which helps retain its transparency. Compactness of stromal fibers also prevents corneal vascularization. In corneal edema, compactness gets lost while in inflammatory conditions, this barrier is broken, both leading to corneal vascularization as shown in Figure 3. Clinical examples being corneal decompensation and post infectious or post injury corneal scar with vascularization (Figure 3).
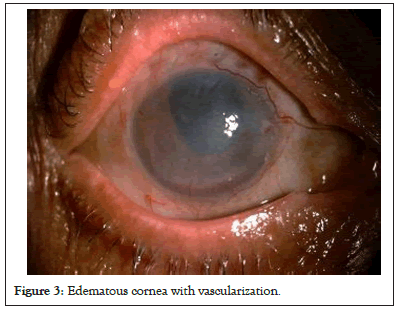
Figure 3: Edematous cornea with vascularization.
The anterior stroma has interwoven collagen fibrils and so has more tensile strength compared to the posterior stroma. As Laser Assisted In situ Keratomileusis (LASIK) refractive surgery involves cutting a flap in the anterior stroma, which is the stronger zone, post-LASIK ectasia can sometimes ensue. Collagen cross linking in keratoconus can increase the biomechanics of the cornea and thus stabilizes the disorder [8]. This treatment strengthens the anterior part of stroma to prevent progression of the disease.
Dua’s layer: The Dua’s layer was identified in 2013 as a pre- descemetic membrane. It is a 5-16 microns thick layer, comprising of 5-11 compact collagen bundles (types 1, 4 and 6). It is a transparent, pliable and resilient structure [9].
Deep Anterior Lamellar Keratoplasty (DALK) is a type of corneal grafting done for advanced keratoconus. It increases the tensile strength of cornea in cases where collagen cross linking is not possible or has failed. In eyes with stromal pathology DALK is increasingly becoming the procedure of choice. The most popular method for this procedure is the Anwar’s Big Bubble (BB) technique [10]. The Dua’s layer has more tensile strength than the bare Descemet’s Membrane (DM). A big bubble created above the Dua’s layer makes surgery safer without accidental DM perforation because the Dua’s layer left behind confers additional strength to the DM (type 1 bubble). Type 2 bubbles is that which is present between the Dua’s layer and the DM, in which the bare DM gets exposed which makes handling a little more precarious for the surgeon during DALK.
Descemet’s membrane: This layer has an anterior 1/3rd zone which is banded, irregular and of fetal origin. It has a lamellar pattern interconnected by electro-dense nodes. The posterior 2/3rd zone are non-banded, homogenous, amorphous and formed after birth. The peripheral rim of DM is the internal landmark of the limbus, known as the Schwalbe’s line. It has a curling property. Its elastic properties maintain the structure and curvature of cornea. It is a biomechanically strong layer [11].
In the event of corneal edema, the corneal volume increases, this causes folds in Descemet’s membrane due to its elastic properties. Due to its high tensile strength, the Descemet’s membrane is able to resist inflammatory corneal melts even when stromal tissue is destroyed. That’s why in melts due to infections or immune etiology, a descemetocele occurs as shown in Figure 4. This can lead into perforation in case of disease progression or rise in intraocular pressure (Figure 4).
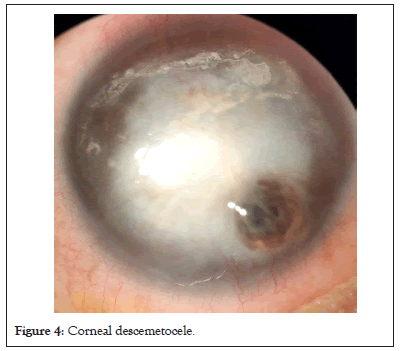
Figure 4: Corneal descemetocele.
Endothelium: This is the single-layered last lamella of the cornea, having hexagonal cells showing a honeycomb-like mosaic. It is derived from neural crest cells and was earlier thought to be non- regenerative. It is a metabolically active layer with abundance of mitochondria and endoplasmic reticulum and no microvilli. Stromal hydration is regulated by a system of pump-leak of ions, chiefly under the control of the corneal endothelium. As the age advances, the number of endothelial cells decline in healthy individuals. Increasing polymegathism (variability in cell size) and pleomorphism (the lack of uniformity of the cell shape) are indications of cell layer distress and remodeling [12].
The hexagonal arrangement of endothelial cells accommodates the maximum number of cells in a given area. Occasional loss of hexagonal cells along with Descemet excrescences seen in the central cornea due to ageing process are called Hassle Henle bodies. These are non-progressive and do not lead to endothelial dysfunction. On the contrary, in endothelial diseases like Fuch’s endothelial dystrophy, there is loss of endothelial cells along with changes in size and shape. This is a progressive condition which can lead to corneal decompensation. Rho-kinase inhibitors like Ripasudil may promote corneal endothelial wound healing [13].
Dimensions of the cornea and applied aspects
It includes curvature, diameter and thickness.
Curvature: The cornea is prolate in shape and is more curved in the center and less in periphery. Average radii of curvature for anterior and posterior curvatures within central 3 mm of optical zone are 7.8 mm and 6.5 mm respectively. The refractive power of the cornea is 40 D-44 D which is 2/3rd of the refractive power of eye. Assessment of the posterior curvature of cornea plays an important role in diagnosing subtle changes in curvature and early corneal ectasias.
The cornea becomes hyperprolate in ectatic conditions like keratoconus, in which central cornea is much steeper than periphery. Increased posterior corneal curvature is considered as an early indicator of keratoconus as shown in Figure 5 [14]. This can even happen without any apparent changes in anterior corneal curvature, at the onset of the disease (Figure 5).
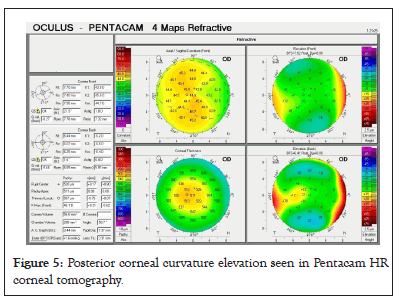
Figure 5: Posterior corneal curvature elevation seen in Pentacam HR corneal tomography.
Diameter: The front part of the adult cornea has a horizontal diameter of about 11.7 mm and vertical diameter of 10.6 mm while the elliptical back part is 11.5 mm in largest diameter. The difference between the horizontal and vertical diameter is due to a greater overlap of sclera and conjunctiva from above and below than laterally.
Increase in corneal diameter is seen in congenital glaucoma because the elastic cornea and sclera are stretched due to rise in intraocular pressure. This gives the classical appearance of large eye or Buphthalmos. In some young patients, glaucoma can cause stretching of the limbus which can be seen as bluish band along the limbus. It signifies increase in intraocular pressure. Distinction between cornea and sclera is not seen clearly in partial or total sclerocornea as shown in Figure 6 [15]. Hence, measurement of corneal diameter becomes difficult in these cases (Figure 6).
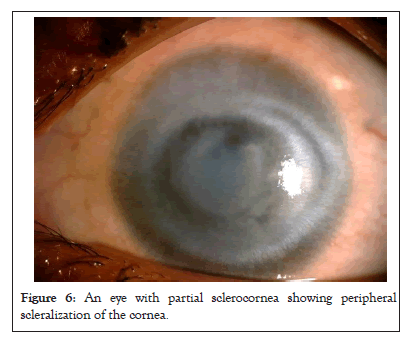
Figure 6: An eye with partial sclerocornea showing peripheral scleralization of the cornea.
Thickness: The Central Corneal Thickness (CCT) is 0.52 mm approximately and peripheral thickness is 0.67 mm approximately. It has been shown that there is diurnal variation in corneal thickness. A study of patients with healthy corneas showed an increase of Intraocular Pressure (IOP) of 0.29 mm of Hg/10 μm of CCT increase in males and 0.12 mm of Hg/10 μm in females [16].
In corneal ectatic disorders, the central cornea is very thin compared to the peripheral cornea. This abrupt change in thickness is reflected in percentage thickness increase which is one of the parameters to diagnose ectasias. Rather than absolute values of central pachymetry, the ratio of central to peripheral pachymetry may be a useful indicator of corneal decompensation in Fuch’s endothelial dystrophy as shown in Figure 7 [17]. Corneal thickness factor and its diurnal variation must be adjusted while measuring intraocular pressure, even in normal subjects (Figure 7).
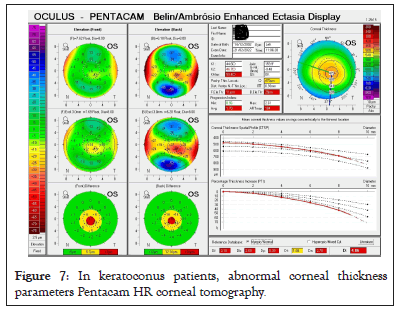
Figure 7: In keratoconus patients, abnormal corneal thickness parameters Pentacam HR corneal tomography.
Neurovascular supply of cornea
It includes nerve supply, blood vessels and corneal lymphatics.
Nerve supply: This is derived from the long ciliary nerves which are branches of the nasociliary nerve which is a branch of the ophthalmic division of the trigeminal nerve. These are unmyelinated nerves. They form three nerve plexuses, namely peri-corneal nerve plexus, sub-epithelial nerve plexus and intraepithelial nerve plexus [18].
The corneal nerves are important for sensations which include touch, temperature and chemical sensations. Intactness of nerves is also important for corneal epithelial attachment. Corneal epithelial migration parallels the direction of the superficial nerve plexus. When corneal sensations are altered in diseases like viral keratitis or trigeminal nerve damage, the neurotrophic factor causes corneal epithelial disturbances leading to persistent epithelial defects (Figure 8) [19].
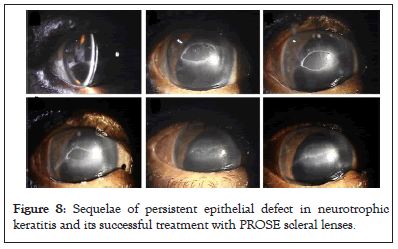
Figure 8: Sequelae of persistent epithelial defect in neurotrophic keratitis and its successful treatment with PROSE scleral lenses.
Blood vessels: The normal cornea is avascular which gives it an immune privilege. Two main types of neovascularization can happen as a response to corneal inflammation. The first is a superficial, arborizing in pattern, bright red in color, sub- epithelial in location. The continuity of the vessels can be traced beyond the limbus along with the conjunctival vessels. The second type is deep having 3 sub-types such as terminal loops, brush and umbel types. They are ill defined and abruptly end at the limbus and have a diffuse red blush.
Corneal angiogenesis occurs as a sequel to an insult to the cornea as it brings with it the cells of immunity as well as repair and aids in flushing the toxins out. However, because these blood vessels are formed in haste in response to the situation, they are immature and leak lipid and the vessels also overstay their time. This ultimately results in loss of transparency, lipid keratopathy and immunogenicity of the cornea.
Treatment of corneal neovascularization is done to prevent corneal graft rejection. It depends on the stage and type of vessels. Topical steroids work well in the inflammatory or pre-neovascularization stage. During early stages of neovascularization, when immature vascular endothelium begins to form, there is a role for Anti- Vascular Endothelial Growth Factor (Anti-VEGF) agents to stop this. For established blood vessels, definitive vaso-occlusive treatments like argon laser photocoagulation and fine needle diathermy are indicated (Figure 9) [20].
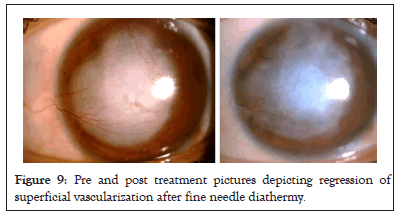
Figure 9: Pre and post treatment pictures depicting regression of superficial vascularization after fine needle diathermy.
Corneal lymphatics: The normal cornea is devoid of lymphatics. Afferent lymphatics are suppressed which contributes to corneal immune privilege, enabling the extraordinary success of histologically incompatible corneal transplantation [21]. The unseen enemy in corneal angiogenesis is the evolution of the lymphatic system with its afferent and efferent arms emanating from the lymph nodes. The lymphatics police the graft antigens, mount an immune response and lead to graft rejection. So, we would like to get rid of new vessels as much as possible before any corneal transplant.
This article looks at the structural characteristics of the cornea including its lamellar arrangement, anterior and posterior curvatures, asphericity, avascularity and such. These have a bearing on the clinical manifestation of corneal diseases. The multi-layered human cornea is an exquisite tissue which has the unique attributes of transparency, avascularity and immune privilege. Correlating the anatomic arrangement of its individual components helps to understand and unravel common clinical diseases of the cornea and aids better teaching as well.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Natarajan R, Toshniwal N (2023) Corneal Structure and Disease: Correlating the Corneal Anatomy with its Clinical Pathology. J Clin Exp Ophthalmol. 14:951.
Received: 28-Jun-2023, Manuscript No. JCEO-23-23420; Editor assigned: 30-Jun-2023, Pre QC No. JCEO-23-23420 (PQ); Reviewed: 14-Jul-2023, QC No. JCEO-23-23420; Revised: 21-Jul-2023, Manuscript No. JCEO-23-23420 (R); Published: 31-Jul-2023 , DOI: 10.35248/2155-9570.23.14.951
Copyright: © 2023 Natarajan R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.