
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Review Article - (2020)Volume 10, Issue 3
The world is battling a pandemic caused by the newly emerged strain of coronavirus; upon identification SARSCoV-2 and in interim named nCOVID-19 by the World Health Organization (WHO). The contagion has sparked a war against humanity with the current global toll of 1,12,241 human lives. Till date no definitive treatment against SARS-CoV-2 has been established. Similar picture of lack of licensed definitive therapy can be traced back to Ebola outbreak and the WHO directed for considering convalescent plasma (CP) therapy for its control. The history of CP as therapeutics dates back to 20th century which renders a scope for consideration in the management of nCOVID-19. Experience from prior outbreak of SARS-CoV-1 has shown that convalescent sera contain neutralizing antibodies to the relevant virus. The core principle of this passive antibody therapy is based on retrieving virus neutralizing antibodies from recovered patients with all ethical considerations and using it as prophylaxis in exposed cases or as therapy in infected patients. It is more effective as prophylaxis than as a treatment modality for the disease. However, when used in early phase of disease, the evidences have reported a decrease in mortality. Cocktails with monoclonal antibodies have also been reported beneficial, but calls for establishing pros and cons in further detail. The sole objective of this review article is to explain how and why the convalescent plasma can serve as a plausible therapeutic modality. In addition, it renders a bird’s eye view on current clinical trials for the same.
Coronavirus; nCOVID-19; Convalescent Plasma; SARS-CoV-2; Pandemic
ACE: Angiotensin Converting Enzyme; ADE-Antibody Dependent Enhancement; ARDS: Acute Respiratory Distress Syndrome; CP: Convalescent plasma; EBOV: Ebola Virus; FDA: Food and Drug Administration; HBIG: Hepatitis B Immunoglobulin; HBIG: Human Rabies Immunoglobulin; mAb: Monoclonal Antibody; IVIG: Intravenous Immunoglobulin; MERS: Middle East Respiratory Syndromerelated coronavirus; NAT: Neutralising Antibody Titre; RSV: Respiratory Syncytial Virus; SARS: Severe Acute Respiratory Syndrome; TMPRSS: Transmembrane Protease Serine; TTI: Transfusion Transmitted Infection; TRALI: Transfusion Related Acute Lung Injury; WHO: World Health Organisation
The emergence of SARS-CoV-2 (nCOVID-19), a new strain of coronavirus has been marked as the third intrusion into the human population following SARS-CoV-1 and MERS-CoV respectively. The tormenting waves of SARS-CoV-2 contagiosity were no longer confined to the boundaries of the Wuhan city of China and this outbreak was soon declared as a ‘Public Health Emergency of International Concern ’ by the World Health Organization [1]. The number of cases continued to spike, widening its horizon of infectivity across 114 countries. This scenario startled the World Health Organization, which later declared it as a pandemic on the 11th of March 2020. In the interim, the virus was recognized to belong to the beta subfamily of coronavirus [2]. The genomic data explicably identified bats as reservoir host and the origin of SARS-CoV-2 was speculated as either due to natural selection in an animal host before the zoonotic transfer or natural selection in humans following the zoonotic transfer [3]. Recent studies have identified pangolins as the intermediate host [4]. Further, the comparative studies corroborate that it didn’t emerge as a product of laboratory manipulation [2].
The spectrum of symptomatic infection ranges from mild to critical; most infections are not severe. The symptoms usually appear 2-14 days after viral exposure which includes fever, cough, shortness of breath and pneumonia. The mortality of critically ill patients with SARS-CoV-2 pneumonia is considerable. This novel nCOVID-19 sparks a biological war between the SARS-CoV-2 contagion and the immune molecules enacting as a potent army of the body resulting in ARDS because of the cytokine storm phenomenon [5]. Older patients (>65 years) with comorbidities and those patients who progress to ARDS are at an increased risk of death. Currently, the global confirmed cases are 18, 07,939 confirmed with 1,12,241 toll of human life (12th Apr.2020) [6].
Till date, neither an effective vaccine nor any anti-viral therapeutic agent has been approved to manage the nCOVID-19 infection. The management of patients mainly focuses on the provision of supportive care, such as oxygen supplementation in mild cases and ventilation, extracorporeal membrane oxygenation for the critically ill patients. Some drugs and therapeutic approaches are still under investigation, including remdesivir, favipiravir, lopinavir/ritonavir, chloroquine/ hydroxychloroquine, IL-6 pathway inhibitors and convalescent plasma.
The use of convalescent plasma is not a new concept. The therapeutic potential of convalescent sera has been well recognized and was used to stem outbreaks of viral diseases such as poliomyelitis [7], measles [8], and mumps [9]. Based on the prior experience and existing evidence in treating other viral infections, the early administration of convalescent plasma or hyper-immune immunoglobulin from patients containing significant antibody titres is likely to reduce the viral load and disease mortality. Over the past two decades, this therapy was successfully used in the treatment [10] of SARS-CoV-1, H5N1 avian influenza [11] and H1N1 influenza [12] wherein the transfusion of convalescent plasma was found to be both effective and safe.
In 2014, the use of convalescent plasma collected from patients who had recovered from Ebola virus disease was recommended by WHO as an empirical treatment during the outbreaks [13] nCOVID-19 convalescent plasma is currently being studied as a therapy for nCOVID-19 patients. Preliminary data from China and other countries suggests some potential promise and further study is needed to determine its efficacy. The US FDA is accepting emergency Investigational New Drug Applications for the use of plasma from recovered patients to treat people who are critically ill with nCOVID-19 [14].
One of the effective passive therapeutic approaches during an outbreak of any infectious disease is the passive antibody therapy from convalescent patients sera who have recovered from the infection.This can be used for the treatment of patients who contract the infection in future. This type of passive therapy is simple but potentially a very effective tool for developing immediate immune responses under critical conditions. In case of the current nCOVID-19 pandemic (SARS-CoV-2), the patients with resolved SARS-CoV-2 viral infection will develop significant serum antibody response (IgG) to different viral epitopes of the SARS-CoV-2 virus and some of these developed antibody responses in the host system will be likely to have the potential to neutralize the virus, as shown in Figure 1. The high level of antibody titres produced by the host immune system against the SARS-CoV-2 virus significantly reduces the chances of getting reinfected.
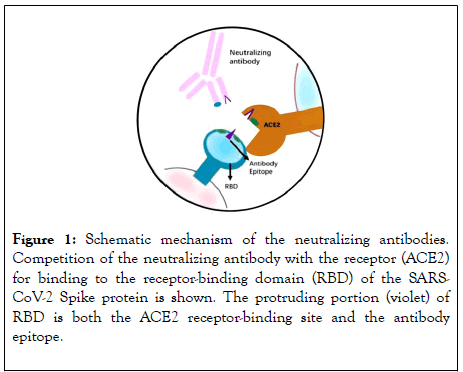
Figure 1: Schematic mechanism of the neutralizing antibodies. Competition of the neutralizing antibody with the receptor (ACE2) for binding to the receptor-binding domain (RBD) of the SARSCoV- 2 Spike protein is shown. The protruding portion (violet) of RBD is both the ACE2 receptor-binding site and the antibody epitope.
Hence, patients who have recovered from SARS-CoV-2 infection can donate their plasma which can be effectively transfused to treat nCOVID-19 infections in other patients as shown in Figure 2 [15]. As plasma transfusion therapy is already being used in many other medical conditions in general and it being simple with no major adverse reactions can be easily utilised in treating patients with nCOVID-19 infection.
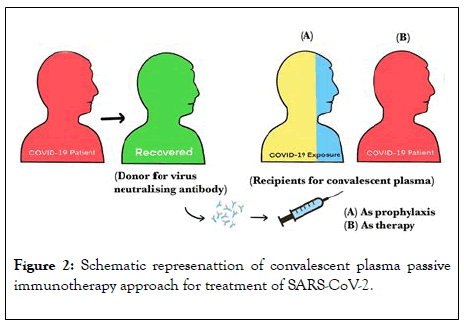
Figure 2: Schematic represenattion of convalescent plasma passive immunotherapy approach for treatment of SARS-CoV-2.
The SARS-CoV-2 pandemic health crisis continues to be an unaccomplished challenge because new cases are being diagnosed, increasing the patient pool. Those patients who have recovered from SARS-CoV-2 infection can be effective serum donors to make antisera for treating SARS-CoV-2 patients. The antisera are stored and can be used to treat at a later date. The potency of antiviral effects of convalescent patient sera, moreover, would have higher significant variability making it less ideal [16].
After the onset of infection, the first antibody to be produced is IgM by day-7 and are detectable in serum till day-21. IgG antibody production starts at around day-14 and continues for a longer time (Figure 3). For convalescent plasma therapy, plasma rich in IgG antibody is usually collected after 14 days from the date of recovery and the first high antibody titres can be observed in recovered patients serum on 21stday because the immune system starts developing antibodies from the day 0 of exposure to the antigen (SARS-CoV-2) and a rise in antibody production can be observed by 14th day. Upon further antibody development in the host immune system by 21st day, a significant rise in these specific antibodies (raised against nCOVID-19) in the serum of the recovered patients can be determined and hence, it can be recommended to use the plasma collected during this peak of antibody increase period of time for the therapeutic purposes effectively.
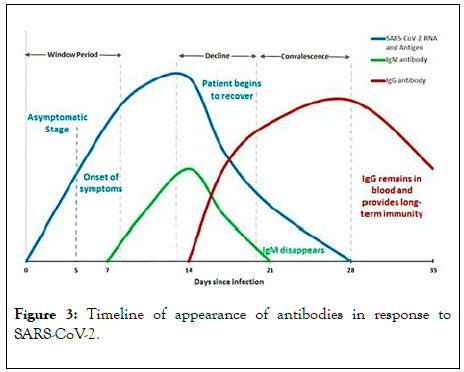
Figure 3: Timeline of appearance of antibodies in response to SARS-CoV-2.
Interestingly, despite initial treatment with oral antiviral drugs and steroidal agents for SARS-CoV-1 case, the chances of patient having an exacerbation of this viral infection have been noticed in the second week of treatment. After aggressive immunomodulatory therapy for SARS-CoV-1, the patient showed an improvement, which further led to complete recovery from the infection effectively [17]. Also a study done by “THE PREVENT” group has reported that fever subsided upon the administration of convalescent plasma containing specific immunoglobulins which has been studied for respiratory syncytial virus infection among hospitalized premature infants and in infants with bronchopulmonary dysplasia using the respiratory synctial virus immunoglobulin prophylaxis. Hence, this can be a supporting data for the convalescent plasma therapy to be admistered against the respiratory viral infections effectively [18].
The understanding of effectivity of convalescent plasma can be substantiated from the past episodes of managing SARS-CoV-1 outbreak which renders a view of reducing the viral load in the host system by administering immunoglobulin [19]. This can be extrapolated for SARS-CoV-2 and can be viewed as the best way of increasing effectivity in terms of higher rate of patient recovering from disease in shorter span of time amidst the scenario of unavailability of specific vaccines.
There are many reports of recent successful experiments- based on the use of convalescent plasma as immunotherapy which includes MERS, Zika viruses, human cytomegalovirus, influenza, respiratory syncytial virus, rabies and Ebola viruses, wherein antibody administration which has shown promise both experimentally and clinically [20]. Production of such convalescent plasma is dependent completely on the presence and willingness of the SARS-CoV-2 survivors to provide plasma for treating other infected patients. The nature of the monoclonal antibody (mAb) cocktails are advantageous as a defined dose can be administered to a patient. However, there are concerns with mAb therapies, as also they are currently limited by production means and the potential of generating escape mutants could eliminate its effectiveness. Large scale production can be relatively easier and polyclonal antibodies (serum) would contain several non-neutralizing and neutralizing antibodies that would considerably reduce the potential for escape mutants [20].
Currently, there are no approved vaccines or any specific antiviral drugs targeting SARS-CoV-2 infection. In 2020, Duan et al., reported that 10 patients, whose severity was confirmed by real-time viral RNA test, were enrolled prospectively. One dose of 200 mL of convalescent plasma (CP) derived from recently recovered donors with neutralizing antibody titres above 1:640 was transfused to the patients as an addition to maximal supportive care and antiviral agents. The primary end-points were the safety of CP transfusion. The second endpoint was the improvement of clinical symptoms and laboratory parameters within 3 days after CP transfusion. This study showed CP therapy was well tolerated and could potentially improve the clinical outcomes through neutralizing viremia in severe nCOVID-19 cases. The optimal dose and time point, as well as the clinical benefit of CP therapy, needs further investigation in larger well-controlled trials [21]. Since an effective vaccine and specific antiviral medicines are unavailable, there is an urgent need to look for an alternative strategy for nCOVID-19 treatment, especially for severe nCOVID-19 infections. Convalescent plasma (CP) therapy, a classic adaptive immunotherapy, has been applied to the prevention and treatment of many infectious diseases for more than a century [22].
Transfusion medicine services should now certainly pursue convalescent patient sera as the right option for treating the infected patients and for significant recovery. Though the mechanism of action and the timing of convalescent plasma in SARS-CoV-2 has to be studied further, immunotherapeutic treatment with CP would be promising with potential benefits as a life-saving treatment of SARS-CoV-2 infected patients.
Emerging trend of clinical trials using convalescent plasma for nCOVID-19 treatment
The entire world is actively looking for medical countermeasures to treat and prevent nCOVID-19. Based on the recent outbreak experiences, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERSCoV epidemic, the plasma rich in neutralizing antibodies (hyperimmunoglobulin) derived from human blood donated by individuals who have recovered from the recent viral infection has proved to be one of the reliable treatment options [23].
This convalescent plasma can be used as antiviral prophylaxis and also for treating individuals diagnosed with nCOVID-19 (Figure 4). Although promising, there are limited clinical data to suggest the beneficial therapeutic effects of convalescent plasma on nCOVID-19. So, it becomes essential to evaluate this in the context of a clinical trial before offering convalescent plasma to nCOVID-19 patients at hospitals.
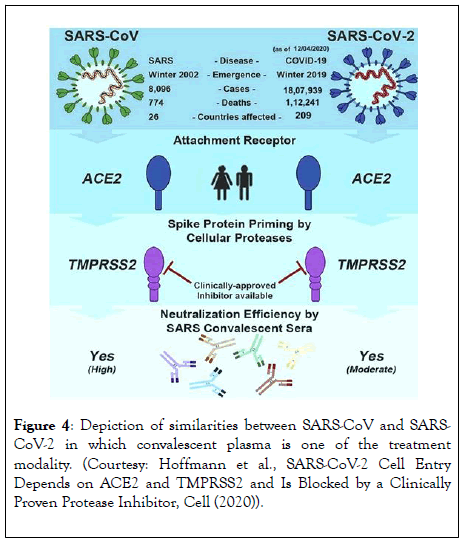
Figure 4: Depiction of similarities between SARS-CoV and SARSCoV- 2 in which convalescent plasma is one of the treatment modality. (Courtesy: Hoffmann et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell (2020)).
As of now on 9th April 2020, almost 10 studies have been registered with clinicaltrial.gov for using blood-derived hyperimmune immunoglobulin to treat nCOVID-19 patients. These studies are mainly from China and the US.
A team lead by Taisheng from Peking Union Medical College Hospital in China registered a clinical trial to evaluate the efficacy and safety of intravenous immunoglobulin (IVIG) treatment for severe n-COVID-19 patients. IVIG obtained from convalescent plasma will be administered to identify patients with severe nCOVID-19 symptoms, with a dosage of 0.5g/kg/day for 5 days. The outcome of clinical improvements will be observed based on several key points, including Murray lung injury score, duration of mechanical ventilation support, and RT-PCR [24]. Another research group in Shanghai Public Health Clinical Centre is actively carrying out a pilot study to show the efficacy of convalescent plasma against SARS-CoV-2 [25].
In a small study led by Xiang, planning to enrol 10 participants at Wuhan Hospital China, proposes to get the anti-SARS-CoV-2 immunoglobulins from convalescent plasma using the immunoadsorption method. This method has the advantage of getting high titre immunoglobulin against SARS-CoV-2. The overall aim of this study is to provide a new strategy for the treatment of nCOVID-19 pneumonia [26].
In yet another clinical trial with 150 participants registered from Johns Hopkins is to evaluate the efficacy of high titre anti-SARSCoV- 2 plasma wherein they intend to examine the efficacy of anti- SARS-CoV-2 plasma upon non-immune plasma. The study illustrates that several parameters will be checked periodically for 90 days, which includes RT-PCR and titering efficacy of immunoglobulin. If conducted successfully, this study will be one of the direct evidence to show the therapeutic potential of the convalescent plasma against SARS-CoV-2 [27].
Another pilot study proposed from Columbia, based on transfusing plasma from recovered patients to patients infected with nCOVID-19 aims to evaluate the correlation between levels of IgM and IgG with the viral load, thereby developing a better understanding of the role of passive and acquired immunity in fighting against the SARS-CoV-2 [28]. A study from Iran actively investigates the effect of convalescent plasma by studying its effects on various immune components like IL-6, C-reactive protein, TNF-α of the patients [29].
Baylor Research Institute registered a clinical trial with 115 participants testing positive for SARS-CoV-2. The participants will typically receive 1 to 2 units of ABO matched donor plasma, which has the anti-SARS-CoV-2 antibody of titre>1:64. Further, the clinical improvements of the treated participants will be monitored carefully, and the results will be objectively analyzed to support plasma therapy [30].
Investigators from San Jose Hospital in Mexico are planning to use convalescent plasma, obtained from the individuals who recovered from SARS-CoV-2 infection and symptom-free for not less than 10 days. Identified patients with severe nCOVID-19 symptoms will then receive this plasma along with the conventional treatment, which includes hydroxychloroquine, azithromycin, and other prescribed antivirals. Key parameters like heart failure, allergic reaction, pulmonary edema, lung infiltration, and SARS-CoV-2 viral load will be evaluated continuously for the following 14 days to examine the safety of the treatment [31].
Significance of convalescent plasma in SARS, H1N1, MERS-CoV and Ebola
The beneficial precedent history of using convalescent plasma for managing the severe acute respiratory infections of viral aetiology optimistically renders the scope of consideration in the management of the nCOVID-19.
SARS (2002-2004): A retrospective case comparison study showed a CFR reduction after plasma treatment which reached statistical significance (absolute reduction in CFR of 23%; 95% CI 6% to 42%; p=0·049) [32]. A second study with a comparator group described a cluster of 29 SARS-CoV cases, where one patient received convalescent plasma and survived (absolute reduction in CFR 7%; 95% CI -2% to 17%; p=0·93) [33].
H1N1/09 (2009-2010): Hung et al. carried out a prospective cohort study where patients received a single 500ml dose of convalescent plasma with a neutralising antibody titre (NAT)>1:160. Univariate analysis showed a significant absolute reduction in CFR of 35% (95% CI 14% to 56%; p=0·01) after treatment. Multivariable analysis also showed a significant reduction in relative risk of mortality (odds ratio [OR] 0·20; 95% CI 0·06 to 0·69; p=0·011) [12].
MERS-CoV (2012): A meta-analysis of studies on the effectiveness of convalescent plasma for treating severe acute respiratory infection revealed that the early use of convalescent plasma after the onset of symptoms is associated with a reduced death rate [33].
Ebola Virus (2013-2016): Ebola Virus (2013-2016): Under the scenario of lack of licensed therapeutics, the World Health Organization approved for controlled clinical studies to study the safety and efficacy of the convalescent plasma and further to serve as a first therapeutic option against EBOV [34,35]. This was partly based on evidence report of 1999 from the Democratic Republic of Congo highlighting the survival of seven EBOV patients out of 8 following infusion of convalescent whole blood [36]. J. van Griensven et al. in his nonrandomized, comparative study including 88 patients provided data showing that the dose of neutralizing antibodies was low and further there was no association between the dose of neutralizing antibodies and human survival [37].
Passive immunotherapy is more effective as prophylaxis than as a treatment. As a therapeutic agent, it has to be administered shortly after the onset of symptoms. The reason for variable efficacy in relation to time has not been clearly understood, but it may be due to the small size of the inoculum at the onset of the disease which is easy to neutralise [38]. Moreover, antibodies work by modifying the inflammatory response, which is also more easily achieved during the initial immune response, a stage that may be asymptomatic [39]. As an example, to emphasize the same, passive antibody therapy for pneumococcal pneumonia was most effective when administered shortly after the onset of symptoms, and there was no benefit if antibody administration was delayed past the third day of disease [40].
Risk-benefit analysis
Prophylactic passive immunotherapy is a well-established modality to prevent disease in those individuals who are exposed to the infective agent or at risk of infection. For example, Hepatitis B immunoglobulin (HBIG), human rabies immunoglobulin (HRIG) and respiratory syncytial virus (RSV) immunoglobulin. Based on the historical experience, it can be anticipated that antibody administration would be more effective in preventing disease than in the treatment of established disease [10].
Risks of passive administration of convalescent sera can be categorized into two groups
Known risks: Transfusion transmitted infections (TTI), Immunological reactions like serum sickness; Transfusion related acute lung injury (TRALI) [41].
Theoretical risks
Antibody dependent enhancement of infection (ADE): There is a theoretical concern that antibodies to one type of coronavirus could enhance infection to another viral strain [42]. It may be experimentally possible to predict the risk of ADE for SARSCoV- 2, as proposed for MERS [43]. The available evidence from the use of convalescent sera in patients with SARS-1 and MERS [23], and anecdotal evidence from its use in 245 patients with nCOVID-19 [43], suggest it is safe but has to be used with utmost caution.
Reinfection: Administration of CP to those exposed to SARSCoV- 2 may prevent disease in a manner that attenuates the immune response, leaving such individuals vulnerable to subsequent reinfection. In this regard, passive antibody administration before vaccination with respiratory syncytial virus was reported to attenuate humoral but not cellular immunity [44].
Risk-benefit assessment must be conducted to assess individual variables so as to decrease the adverse reactions.
FDA Approval for convalescent plasma therapy
The US Food and Drug Administration has approved the emergent use of plasma as an investigational new drug from recovered patients to treat people who are critically ill with nCOVID-19, provided that doctors get an approval over the telephone [2]. The FDA has also instructed doctors wanting to study the use of convalescent plasma to follow the usual system for an investigational new drug (IND) application.
The plasma of those patients with no symptoms for 14 days, tested negative for nCOVID-19 and who can donate blood is collected. The FDA said that it was providing emergency access The plasma of those patients with no symptoms for 14 days, tested negative for nCOVID-19 and who can donate blood is collected. The FDA said that it was providing emergency access
Severe nCOVID-19 disease is defined as
a. Dyspnoea,
b. Respiratory frequency ≥ 30 breaths per minute,
c. Blood oxygen saturation ≤ 93%,
d. Ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2)<300, or
e. Lung infiltrates>50% within 24 to 48 hours.
Life threatening disease is defined as respiratory failure, septic shock, or multiple organ dysfunction or failure. In such cases, doctors can submit a form online or call FDA ’ s hotline telephone number (1-866-300-4374) to get verbal approval for treatment, which is promised within four to eight hours [45].
Battling a pandemic is a big challenge because of the nonavailability of definitive therapy and the immense time required to come up with an effective vaccine. In such scenarios, passive immunotherapy, particularly convalescent plasma has been a lifeboat to combat pandemics.
Convalescent plasma is a promising treatment for patients with early symptoms and to prevent disease in those who are exposed to nCOVID-19. Rapidly expanding nCOVID-19 patient pool is an opportunity to perform clinical studies on the efficacy of CP against this viral agent. It has been postulated that convalescent serum will prevent SARS-CoV-2 infection to whom it is administered. If this is established through well-structured trials and if the situation demands, healthcare workers exposed to nCOVID-19 can be prophylactically treated with CP instead of quarantining them for the effective utilization of health care services.
nCOVID-19 pandemic, spreading like wildfire compels us to address it through the promising convalescent plasma therapy by mobilising the resources effectively. Trained professionals are required for effective collection of CP from recovered patients after proper consent and its proper storage. It demands a focused multi-speciality team to put Convalescent plasma therapy into clinical practice to combat this pandemic. Largescale randomized clinical trials are the need of this hour to demonstrate and establish the target population for therapy, the efficacy of CP and the optimal time, dose and duration of treatment which could help change the impact and course of this pandemic.
We thank Dr Naveen Jeyaraman, Junior Resident of Orthopedics, Kasturba Medical College, Manipal, Karnataka, India, DrPrajwal GS, Junior Resident of Orthopedics, JJM Medical College, Davangere, Karnataka and DrMadhurya S, Junior resident of Dermatology, Raja Rajeshwari Medical college, Bangalore, India for literature search regarding nCOVID-19. All authors have contributed equally in writing and proofing the script.
OCRD Numbers: AnudeepTalagavadiChannaiah – 0000-0002-9954-5179, MadhanJeyaraman – 0000-0002-9045-9493, Dharma U Shetty–0000-0002-4254-1214, Hemmanth Raj M – 0000-0002-9590-1690, Ajay SS – 0000-0001-8905-3245, RajeswariSomasundaram – 0000-0001-9062-3815, Vinodh Kumar V–0000-0002-4974-9317, Rashmi Jain – 0000-0003-4386-6755, ShirodkarJasw and Dilip-0000-00015287-9633.
Citation: AnudeepTC, Jeyaraman M, Shetty DU, Raj MH, Ajay SS, Somasundaram R, et al. (2020) Convalescent Plasma as a Plausible Therapeutic Option in nCOVID-19: A Review. J Clin Trials 10:409. doi: 10.35248/2167-0870.20.10.409
Received: 09-Apr-2020 Accepted: 22-Apr-2020 Published: 29-Apr-2020 , DOI: 10.35248/2167-0870.20.10.409
Copyright: © 2020 Anudeep TC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.