
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Review Article - (2020)Volume 10, Issue 2
Skin and soft tissue infections (SSTIs) are common clinical conditions ranging from mild superficial/cutaneous legions to life-threatening disseminated infections. Roughly 15% of the patients that seek medical intervention have a skin lesion or disease which is infectious. The emergence and rapid spread of antimicrobial resistance has complicated the therapy and negatively impacted patient outcomes in cases of SSTIs. This study serves to highlight the most common causative agents of SSTIs in Jamaica based on frequency of isolation, their drug resistance, and also their frequency associated with the different demographic groups during the time period covered. Data pertaining to culture and sensitivity of SSTIs done between 2012 and 2015 was collated from the main reference lab in Jamaica, with permission from Ministry of Health (MOH), and analyzed with the IBM SPSS 25 system.
The patients included 139 females, 163 males and 75 of unknown gender. The order etiological agents causing skin and soft tissue infections in Jamaica closely mirrors the order reported in North America, Latin America and Europe with S. aureus being the most prevalent followed by various Enterobacteriaceae, P. aeruginosa and β-hemolytic Streptococci. This study showed that 77.1% of the SSTI isolates were resistant to at least one drug while 18.8% were deemed to be multidrug-resistant (MDR) and one case of extensive drug-resistance (XDR) was noted in 2012. The frequency of overall drug resistance and MDR isolates increased from 2013 to 2015. With the etiology of SSTIs in Jamaica mirroring global trends, it critical that we pay close attention to current global trends and recommendations concerning the management of SSTIs in order to improve patient outcomes.
Skin and soft tissue infections; Females; Multidrug-resistant
The skin is the largest organ of the body and serves as the barrier protecting the internal organs from the external environment. Any breach in the skin creates a portal of entry for microorganisms which then grow and multiply causing infections and inflammation [1]. Skin and soft tissue infections (SSTIs) are common clinical conditions ranging from mild superficial/cutaneous legions to life-threatening disseminated infections [2]. SSTIs are clinical manifestations frequently associated with vasculopathy, immunosuppression, decreased lymphatic drainage or neuropathy [3]. SSTIs can be caused by fungal, viral, parasitic, and bacterial agents with the most common and focus of this study being the bacterial and fungal agents.
SSTIs pose a global threat and health problem to the general public [4]. Roughly 15% of the patients that seek medical intervention have a skin lesion or disease which is infectious [2]. In most recent years, a noteworthy developing pattern of SSTIs, whether community or hospital acquired, shows a steady increase of health care services and socioeconomic burden [5].
Table 1 shows the order of bacteria causing skin and soft tissue infections in North America, Latin America and Europe from 1998-2004.
| Rank | Pathogen | No. of isolates (% of total) |
|---|---|---|
| 1 | Staphylococcus aureus | 2602 (44.6) |
| 2 | Pseudomonas aeruginosa | 648 (11.1) |
| 3 | Enterococcus spp. | 542 (9.3) |
| 4 | Escherichia coli | 422 (7.2) |
| 5 | Enterobacter spp. | 282 (4.8) |
| 6 | Klebsiella spp. | 248 (4.2) |
| 7 | β-Hemolytic streptococci | 237 (4.1) |
| 8 | Proteus mirabilis | 166 (2.8) |
| 9 | Coagulase-negative staphylococci | 161 (2.8) |
| 10 | Serratia spp. | 125 (2.1) |
Table 1: The order of bacteria causing skin and soft tissue infections in North America, Latin America and Europe 1998-2004 (Moet, 2007) [6].
Based on the ranking [6], S. aureus is the most common causative agent of SSTIs and is known to produce a myriad of bacterial toxins and among other highly effective virulence factors. The emergence and rapid spread of antimicrobial resistance has complicated the therapy and negatively impacted patient outcomes in cases of SSTIs. The most frequent drug resistances are Methillicin-resistant S. aureus (MRSA), vancomycin-resistant Enterococcus (VRE), imipenem-resistant P. aeruginosa, and extended spectrum beta-lactamases (ESBL) among E. coli, Klebsiella spp. and P. mirabilis [7].
This study serves to highlight the most common causative agents of SSTIs based on frequency of isolation, their drug resistance, and also their frequency associated with the different demographic groups during the time period covered.
The data received from the main reference lab in Jamaica, with permission from Ministry of Health (MOH), was analyzed using IBM SPSS 25 software. Graphs and tables were constructed to illustrate and highlight the different trends, occurrence, and frequencies in the age, gender, and most common etiological causative agent.
SSTIs represent approximately 6.3% of all specimens submitted to the microbiology department during the period studied with a slight increase in the number of specimens per year (Table 2).
| Year | Number of cases |
|---|---|
| 2012 | 112 |
| 2013 | 130 |
| 2015 | 135 |
| Total | 377 |
Table 2: Number of SSTI specimens processed 2012-2015.
The patients included 139 females, 163 males and 75 of unknown gender. A vast majority of the patients were adults (42.4%) followed by neonates (10.3%), seniors (9.5%), adolescents (7.4%) and young adults (6.9%) (Figure 1).
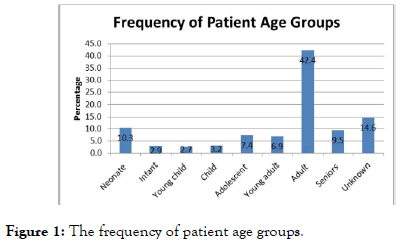
Figure 1. The frequency of patient age groups.
The order etiological agents causing skin and soft tissue infections in Jamaica (Table 3) closely mirrors the order reported in North America, Latin America and Europe (Table 1) [6] with S. aureus being the most prevalent followed by various Enterobacteriaceae, P. aeruginosa and β-hemolytic streptococci. This finding suggests that there might be possible similarities in the pathogenesis and prognosis of the infections caused by these microorganisms. It is therefore necessary for healthcare providers to stay abreast of best practices published in the regions showing similar patterns to inform the management these infections to improve patient outcomes.
| Rank | Microbe | Number of cases | Percent of cases |
|---|---|---|---|
| 1 | S. aureus | 141 | 39.30% |
| 2 | Enterobacter spp | 66 | 18.40% |
| 3 | Proteus spp | 62 | 17.30% |
| 4 | Klebsiella spp | 54 | 15.00% |
| 5 | E. coli | 46 | 12.80% |
| 6 | Pseudomonas aeureginosa | 41 | 11.40% |
| 7 | Acinetobacter spp | 32 | 8.90% |
| 8 | Non hemolytic strep | 26 | 7.20% |
| 9 | Enterococous spp | 19 | 5.30% |
| 10 | Beta-hemolytic strep | 16 | 4.50% |
| 11 | Alcaligenes spp | 8 | 2.20% |
| 12 | Morganella morgarii | 7 | 1.90% |
| 13 | Providencia spp | 7 | 1.90% |
| 14 | Citrobacter spp | 6 | 1.70% |
| 15 | Coagulase-negative Staph | 4 | 1.10% |
| 16 | Alpha-hemolytic Strep | 4 | 1.10% |
| 17 | Hemophilus spp | 4 | 1.10% |
| 18 | Candida spp | 3 | 0.80% |
| 19 | Salmonella spp | 1 | 0.30% |
| 20 | Serratia spp | 1 | 0.30% |
Table 3: The order of bacteria causing skin and soft tissue infections in Jamaica 2012-2015.
When the prevalence of microorganisms is analyzed by groups (Figure 2), each year shows a similar distribution with the majority of isolates being Enterobacteriaceae followed by Staphylococci, non-fermenting GNBs then Streptococci. This finding was contrary to other regions where Staphylococci are the dominant isolates [6]. The primary pathogen affection all age groups except children and seniors was S. aureus. The primary pathogen for children and seniors were P. aeruginosa and Proteus spp. respectively (Table 4). These exceptions were contrary to the findings in literature where S. aureus is the main pathogen in SSTIs in children [8,9] and adults [10].
| Age group | No. 1 isolate | Frequency |
|---|---|---|
| Neonate (0-30 days) | S. aureus | 27.10% |
| Infant (1 month-2 years) | S. aureus | 38.50% |
| Young child (3-6 years) | S. aureus | 46.20% |
| Child (7-12 years) | P. aeruginosa | 27.80% |
| Adolescent (13-18 years) | S. aureus | 45.90% |
| Young adult (19-25 years) | S. aureus | 48.40% |
| Adult (26-64 years) | S. aureus | 23.90% |
| Seniors (65+ years) | Proteus spp | 19.40% |
Table 4: The most frequently isolated etiologic agent among different age groups.
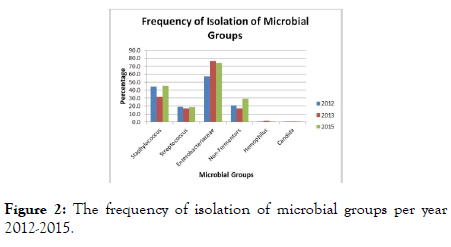
Figure 2. The frequency of isolation of microbial groups per year 2012-2015.
The polymicrobial nature of SSTIs has been well documented [11-15]. This study found that 39.3% of cases were found to have two or more isolates with the maximum number of isolates in any one case being five (Figure 3). Polymicrobial infections can prove challenging to distinguish from specimen contamination due to the fact that most of the etiological agents are normal or transient flora thereby posing a risk of misinterpretation and misdiagnosis which in turn compromises patient management and outcomes. It is crucial then, to ensure the best quality results, that each laboratory adapt a protocol for investigating and distinguishing polymicrobial infections from contamination. Antimicrobial Resistance (AMR) is growing problem on a global scale [6]. Drug resistance has is expressed in varying degrees and therefore definitions have been prescribed for different categories. Multidrug-Resistance (MDR) is defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories, extensive drug-resistance (XDR) is defined as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories and pandrug-resistance (PDR) is defined as non-susceptibility to all agents in all antimicrobial categories. This study showed that 77.1% of the SSTI isolates were resistant to at least one drug while 18.8% were deemed to be MDR and one case of XDR was noted in 2012. The frequency of overall drug resistance and MDR isolates increased from 2013 to 2015 (Table 5). These observations are congruent with global trends where steadily increasing prevalence of AMR is reported.
| Resistance category | Year (%) | Total (%) | ||
|---|---|---|---|---|
| 2012 | 2013 | 2015 | ||
| Drug resistance | 74.9 | 73.4 | 81.9 | 77.1 |
| Multidrug resistance | 22.2 | 13 | 21.3 | 18.8 |
| Extensive drug resistance | 2.4 | 0 | 0 | 0.5 |
Table 5: The frequency of isolation of drug-resistant microbes.
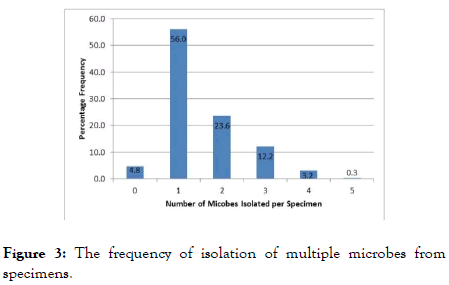
Figure 3. The frequency of isolation of multiple microbes from specimens.
MRSA and VRSA were found to be at an overall prevalence of 14.2% and 10% respectively with neither strain being reported in 2015 (Table 6); this suggests either eradication or false negative tests for MRSA and VRSA in 2015 as the global trends show increasing prevalence[16,17]. Against the background of rising non-susceptibility to vancomycin among S. aureus and the contradicting decrease observed in this study, the protocols being used to detect MRSA and VRSA need to be reviewed and steps taken to ensure the highest sensitivity is maintained to avoid false negative results.
| Microbe | Year (%) | Overall (%) | ||
|---|---|---|---|---|
| 2012 | 2013 | 2015 | ||
| MRSA | 33.3 | 10.8 | 0 | 14.2 |
| VRSA‡ | 12.5 | 0 | 0 | 10 |
| MRSE | 0 | 50 | 100 | 50 |
| VRE | 14.3 | 50 | 100 | 47.4 |
| Carbapenem-Resistant GNB | 17.5 | 1.1 | 6.5 | 7.2 |
Table 6: The frequency of isolation of notable drug-resistant strains.
A steady increase in the prevalence was observed for VRE across the period studied (Table 6). This is consistent with global trends [18,19]. The prevalence of carbapenem-resistant GNBs stood at 7.2% showing an increase between 2013 and 2015. This trend is also consistent with literature as carbapenemase-producing Enterobacteriaceae (CRE) has spread worldwide during the last decades [20]. Of note is the observation that 50% of the isolated coagulase-negative Staphylococcus were found to be methicillin resistant Staphylococcus epidermidis (MRSE). This is however consistent with the emergence of MRSE in other regions with a prevalence of 56.3% reported in one study [21].
Significant resistance was observed to all drug classes tested with over 30% resistance to tetracycline, penicillins, carbapenems and cephalosporins with an increase in resistance reported for each of these drug classes between 2013 and 2015 (Figure 4) which is consistent with the reported increases around the world [6,15].
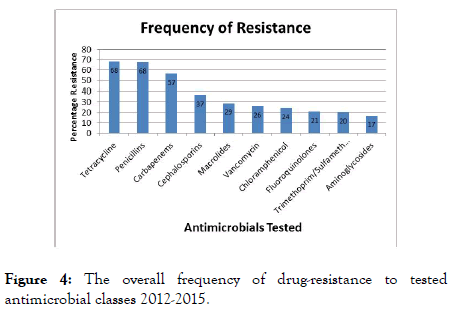
Figure 4. The overall frequency of drug-resistance to tested antimicrobial classes 2012-2015.
The etiology of SSTIs in Jamaica closely mirrors that reported in North America and the rest of the world. We therefore need to pay close attention to current global trends and recommendations concerning the management of SSTIs in order to improve patient outcomes. AMR among the etiological agents of SSTIs is relatively high in all classes of drugs thereby posing a threat to successful patient therapy and increasing the risks of complications. The findings in this research support the need for broader studies into the etiology and AMR trends in other types of infections.
Citation: Robinson D, Pitkin P, Whitely D (2020) Contemporary Causes of Skin and Soft Tissue Infections in Jamaica. J Clin Trials. 10:399. DOI: 10.35248/2167-0870.20.10.399
Received: 25-Feb-2020 Accepted: 10-Mar-2020 Published: 17-Mar-2020 , DOI: 10.35248/2167-0870.20.10.399
Copyright: © 2020 Robinson D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.