Translational Medicine
Open Access
ISSN: 2161-1025
ISSN: 2161-1025
Research Article - (2023)Volume 13, Issue 4
Background: Hospital death is significant in patients with Severe Acute Pancreatitis (SAP). These patients have elevated Intra-Abdominal Pressure (IAP), which may result in organ dysfunction. The goals of this study were to examine the overall incidence of rising IAP in patients with SAP, as well as the progression of organ dysfunction and risk of mortality factors for higher IAP.
Materials and methods: Between 2020 and 2023, 118 cases of acute pancreatitis were managed in the intensive care unit of (University). Patients were categorized into quartiles 1-4. The intra-vesical technique was used to assess elevated IAP levels.
Results: Peak IAP, max Sequential Organ Failure Assessment (SOFA) score, max Acute Physiology and Chronic Health Evaluation (APACHE) II score, top creatinine level and age, with lactate peak level, were substantially related to IAP and were higher in non-survivors. The maximal IAP in retrospective groups 1-4 was 7-14 mmHg, 15-18 mmHg, 19-24 mmHg and 25-33 mmHg, respectively, with hospital mortality rates of 10%, 12.5%, 22.2% and 50%, respectively. Respectively, the difference was statistically significant. The highest quality Intensive Care Unit (ICU) free days were 45.7, 38.8, 32.0 and 27.5 days, respectively. Categories one-four had significant statistical significance.
Conclusion: A greater IAP in SAP cases presented with early organ damage and fewer ICU-free days. IAP was assessed throughout ICU inpatient care to determine the cure rate after decompression.
Compartment syndrome; Mortality rates; Intra-abdominal pressure; Pancreatitis; Organ failure
Despite progress in managing severe acute pancreatitis, hospital deaths remain significant [1,2]. Bacterial virulence, the degree of damaged pancreatic parenchyma and the presence of Multiple Organs (MOF) are leading predictors of death [3]. According to the present study, some people who died of early MOF may have suffered from uncontrolled Acute Compartment Syndrome (ACS). Massive revived fluids early in the disease, combined with a great inflammatory process, subsequent visceral edema, fluid collection and increased Intra-Abdominal Pressure (IAP) [4].
IAP normally ranges from 0-5 mmHg, auscultated with respiration; patients with a pressure of 15 mmHg are more susceptible to organ dysfunction; a pressure of 20 mmHg causes compartment syndrome; pressures higher than 25-30 mmHg is linked to severe acute pancreatitis and organ failure [5-11]. The worst value from each day was used to calculate the Sequential Organ Failure Assessment (SOFA) score, which quantifies the degree of organ dysfunction. Regarding the IAP score, patients were divided into quartiles 1-4 [12,13].
SAP is a condition that routinely involves Intra-Abdominal Hypertension (IAH) and Acute Compartment Syndrome (ACS). According to one study, the incidence of IAH using the original Atlanta criteria was between 60 and 80 percent and ACS developed in approximately 25%-50% of the patients [14,15]. The incidence of severe illness may be even greater with the new criteria because some patients with what is now called moderate pancreatitis were previously classified as severe. A recent comprehensive study highlighted acute pancreatitis as a risk factor for IAH, but multiple additional variables are frequently present in patients with SAP [16].
Numerous investigations of ACS in patients with SAP have been described recently; however, information on this issue is still lacking. This investigation concentrates on the therapeutic management of this dangerous illness because there are still many unanswered problems regarding medical therapy, evidence, execution and interventions.
Over the last few decades, there have been significant breakthroughs in the understanding of the pathogenesis, diagnosis and therapy of IAH and ACS. The relevance of IAH in critically ill patients has been explicitly researched, leading to an improved understanding of the mechanisms of organ dysfunction caused by situated IAP and earlier opportunities for therapies. Furthermore, therapeutic and minimally invasive procedures have been developed and shown in limited research to be beneficial.
For ACS during SAP, there is no accepted medical, surgical or intervention therapeutic cure. The IAH and ACS classifications and therapy guidelines have been released by the World Society of Abdominal Compartment Syndrome (WSACS), but it is unknown if SAP individuals can use these guidelines.
In all reports on this condition, IAH, particularly ACS, has been linked to poorer outcomes [17]. Some even proposed using IAP as a marker of severity in SAP and using a cutoff of 14 mmHg, the receiver operating characteristic curve of IAP was higher than the Ranson and Imrie score, which could make IAP measurement a simple tool [18]. However, no other studies have evaluated IAP for this purpose.
We aimed to examine the overall incidence of rising IAP in patients with SAP, as well as the complications related to organ dysfunction and risk of mortality factors for higher IAP.
Prospective individuals with SAP treated at the ICU Zagazig University Hospital between 2020 and 2023 were identified using the ICU automated database board sheet system and at least one organ malfunction confirmed the diagnosis clinically and by Computed Tomography (CT). In 74 of 118 patients, if IAH was acutely anticipated due to excessive distension of the abdomen paired with either early or deteriorating organ failure, IAP was used to evaluate the following.
Recording of intra-abdominal pressure
At 4-hour intervals, intravenous pressure was recorded in the supine position at the end of expiry using a Foley catheter with a three-way stopcock. The IAP was recorded every 4 h on the day of enrollment and the peak IAH values were obtained. A constant rise in IAP was regarded as a boost of not less than three successive calibrated readings. Patients were divided into two groups (A and B) depending on the presence or absence of IAH (IAH-IAP>12 mmHg) based on their highest IAP values.
Interventional radiological therapy
Intervention procedures appear to be needed when medical assistance for IAH is ineffective and ACS has already manifested. Percutaneous Catheter Drainage (PCD) insertion following radiological guidance ought to represent the initial line of treatment for these individuals to alleviate ACS [19-21]. Milanesi, et al., [20] found that intra-abdominal was significantly associated with drainage system volume, length of hospital stays and APACHE II score in an assigned study assessing the impact of implanted catheters and conservative measures in the management of ACS in patients with intense severe pancreatitis. Participants with abdominal catheters become better than those who received conservative treatment in terms of outcomes (relief of abdominal discomfort and length of hospitalization). It was not significantly different from the rate of fatalities, which reduced from 20.7% to 10%.
Conservative management for all critically ill and ICU cases
Optimize fluid administration: Proper volume therapy is crucial to prevent IAH and ACS in patients with SAP. Obtaining the best possible systemic infusion with appropriate fluid delivery is crucial for treating acute pancreatitis. To re-establish end-organ perfusion in cases of acute pancreatitis, some recommendations advise swiftly augmenting with isotonic crystalloid solution [22]. The person’s susceptibility to volume resuscitation, the duration following the start of pancreatitis (a minimum of 24 h is essential) and the individual’s susceptibility to fluid sequestering should be considered. The intensive care physician is crucial at this stage in reversing hypovolemia and avoiding iatrogenic abdominal compartment syndrome [23]. In response, Malbrein’s team developed the ROSE concept, which divides fluid distribution into four stages such as rescue, optimization, stabilization and release [24].
Following an early enthusiasm for active fluid therapy, it quickly became apparent that intensive resuscitation for more than 48 hours following the start of pancreatitis increases mortality by causing the development of abdominal compartment syndrome [25,26]. Beginning violent fluid administration (a bolus of 20 mL per kilogram of weight of the individual after 3 mL per kilogram per hour) led to an increased likelihood of excess fluids lacking improving clinical results [27,28].
A more individualized approach is necessary because of the danger of under-resuscitation when a predetermined infusion rate is used and the risk of complications when fluid treatments are administered too aggressively. Hemodialysis and loop diuretics should also be taken into consideration [29].
Antibiotics: Due to bacterial translocation and decreased immune function in the early phases, individuals with severe necrosis pancreatitis are more likely to contract the infection and may experience infected pancreatic necrosis 2-4 weeks after the disease first manifests [30]. Due to the possibility of bacteremia, the experimental application of antibiotics is advised in patients who experience organ failure during SAP, rather than the preventative use of antibiotics [31,32]. Antibiotics have been treated as misused and used unduly, contrary to previous advice [33,34]. The chance of infection encountered in hospitals increases when antibiotics are misused. The subset of those who benefited most from timely prescription of antibiotics was identified by the initial increase in inflammatory markers [35,36]. Antibiotics must be administered because individuals who advance to IAH are susceptible to sepsis [37].
Energy nourishment: To preserve the advantages of the transmission of bacteria, widespread infection, organ failure and immortality, it is advised that it should not be postponed for longer than 48 hours. A nasojejunal tube should be used to administer the general diet at an average rate of 10 mL/h, according to recommendations. Since bacterial proliferation and transfer are less likely to occur through the gut, hypertension inside the abdomen is avoided. In individuals with abdominal compartment syndrome, oral feeding should be stopped and complete parenteral nourishment should be started.
All patients received standard care involving nasogastric decompression with various intravenous fluids, analgesics and other complementary prescribed medications. During assistance, antibiotics were administered based on the possibility of sepsis and the existence of culture and sensitivity reports. If necessary, ventilatory aids and vasopressors were administered. Unless recommended, dietary assistance may be enteral. APACHE II score was obtained at the time of admission. The Sequential Organ Failure Assessment (SOFA) score was used to diagnose organ failure. When a substitute conservative technique to minimize IAH was unsuccessful, surgery occurred earlier and subsequently in patients who were not receptive to the boost strategy.
Drugs for intraluminal pressure decline: Sabbagh, et al., [27] recently conducted a randomized controlled trials to gauge the success rate of neostigmine in minimizing IAP in AP with IAH. Neostigmine was administered Intra Muscular (IM) at Bis In Die (BID) and raised to 1 mg every 8 or 6 h if the IAP level fell at a much faster rate in the neostigmine group. Neostigmine was swiftly effective, decreasing IAP 3 h after one injection. After 24 h, the IAP declined by approximately 20%. The neostigmine group had a larger fecal quantity during the 7-14-day monitoring session. By the fourth day, the treated group experienced early pain alleviation and an important reduction in IAP (from 14 mmHg to 6 mmHg) and APACHE II scores.
Other medical administrative of IAH/ACS in AP
Numerous therapeutic techniques for IAH and ACS can be classified based on the basic mechanism that boosts IAP. Other factors are generally associated with these; therefore, such measures are not distinct. Comparably, since IAH is a process, therapies can be either preventative or curative. The degree of endorsement in the mainstream WSACS can, at times, be weak or absent because of early clinical pictures. There have been no trials showing the effects of sedation on IAP. Nevertheless, the WSACS suggestion for appropriate stress and agony in IAH continues. Analgesics are also essential for the treatment of difficult conditions such as Acute Pancreatitis (AP). Cisatracurium injection has been proven to reduce IAP in cases of IAH-attributed water collection, gut injuries or burning. Brief trials of muscarinic therapy have been demonstrated to be secure and efficient in treating critically ill patients, especially IAH [38]. The algorithm of medical treatment is shown in Figure 1. Definitions by Kirkpatrick, et al., [3] to clinician’s guide to types of management in severely ill patients. Table 1 shows the most recent manuscript described and updated in 2013 (Figure 1 and Table 1).
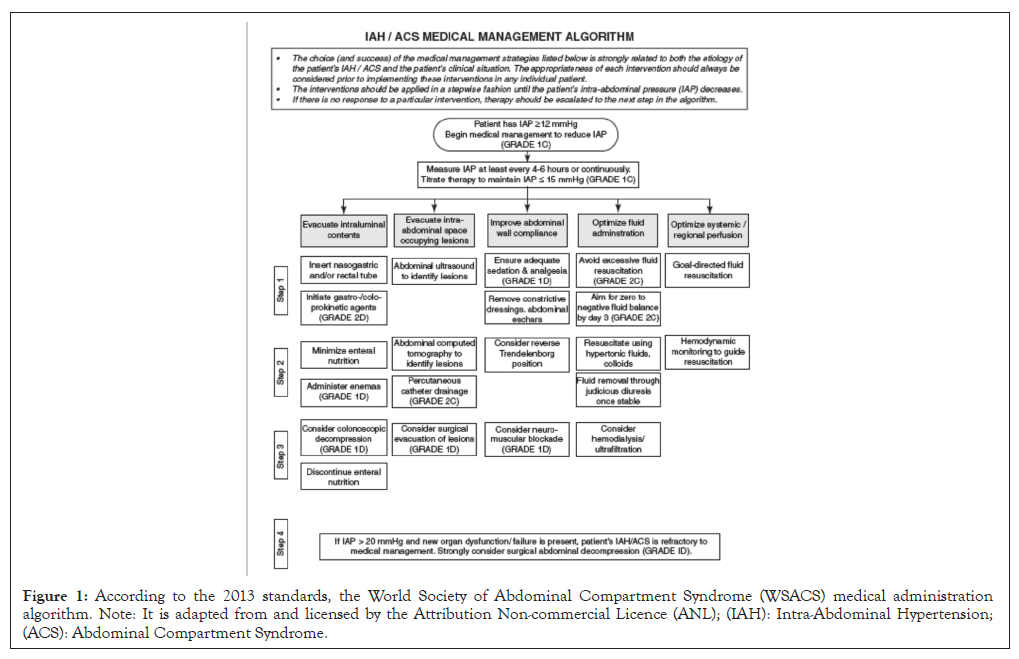
Figure 1: According to the 2013 standards, the World Society of Abdominal Compartment Syndrome (WSACS) medical administration algorithm. Note: It is adapted from and licensed by the Attribution Non-commercial Licence (ANL); (IAH): Intra-Abdominal Hypertension; (ACS): Abdominal Compartment Syndrome.
| S. No | Definition |
|---|---|
| 1 | The steady-state pressure buried within the abdominal cavity is referred to as IAP. |
| 2 | The bladder is used as the source basic for paroxysmal IAP value, with a max infusion amount of 25 ml of saline. |
| 3 | IAP by (mmHg) at the end of expiration in the supine posture, with zero transducers at the mid-axillary line. |
| 4 | In critically unwell people, IAP is around 5-7 mmHg. |
| 5 | A continuous or recurring pathological IAH is defined as an increase in IAP of 12 mmHg. |
| 6 | ACS is known as a continuous IAP of >20 mmHg regardless of the APP of 60 mmHg, which may be correlated with emerging organ dysfunction/failure. |
| 7 | The IAH score is as the following: |
| Grade one IAP 12-15 mmHg. | |
| In grade two, the IAP is 16-20 mmHg. | |
| 8 | Basic IAH, referred to as ACS, is a disorder that involves abdomen or pelvis damage or disease that frequently demands early surgeries or invasive radiological therapy. |
| 9 | Second, IAH, frequently referred to as ACS, is a term used to describe disorders that do not start in the abdominal region. |
| 10 | Recurrent IAH or ACS occurs when IAH or ACS reappears after previous surgical or therapeutic management of the main or second IAH or ACS. |
| 11 | APP=MAP-IAP |
| 12 | When compartmental pressure occurs in multiple anatomical, compart is called a poly-compartment syndrome. |
| 13 | The ease with which the abdominal wall and diaphragm can broaden is termed abdominal compliance. The alteration in IAP per increment in intra-abdominal capacity should be given. |
| 14 | Because the skin and fascia were not connected after the procedure known as la, the open abdomen demands a temporary abdominal closing. |
| 15 | Shifting of abdominis to the lateral side by which the musculature and fascia of the lining of the abdomen, most notably the rectus abdominis muscles and their enveloping fascia, migrate laterally farther from the centerline over time. |
Note: IAP: Intra-Abdominal Pressure; IAH: Intra-Abdominal Hypertension; ACS: Acute Compartment Syndrome; APP: Abdominal Perfusion Pressure; MAP: Mean Arterial Pressure.
Table 1: Definitions in a clinician’s guide to management of IAH and ACS in severely ill patients.
Times of invasive therapy
There is no definite agreement regarding the best surgical intervention for patients with ACS during SAP. There is a lack of information on the best surgical technique, signaling and time for these individuals. Decompressive laparotomy is recommended in instances of explicit ACS compared to strategies that avoid the use of decompressive laparotomy, according to the WSACS clinical practice guidelines. There is no discussion of the best method for laparotomy or what obvious ACS that entails controversy.
The timing of abdominal decompression in the care of these individuals is still unknown. Outcomes unmistakably showed that initial decompression (during the first four days) was associated with significantly fewer deaths than delayed decompression (during the following four days).
In an attempt to enhance the outcomes of patients who develop ACS during SAP, numerous surgical approaches have been outlined in current scholarship. Several documented processes may be helpful and appropriate for such patients. The most popular procedure for treating ACS is decompressive laparotomy with concurrent laparostomy.
Surgical treatment of central-abdominal collection treatments
The volume of abdominal drained fluid was 2900 cc. The rate of cyst formation was also reduced, as was the relief of abdominal discomfort and inpatient time [39].
A gradual decrease in IAP occurred after many days (7-14 days) of pain relief and organ function improvement. Some compared percutaneous catheter drainage with peritoneal lavage in 86 individuals with SAP in a randomized controlled experiment. In fluid-filled toxin and cytokinesis, there was no statistical difference between the two methods, except for the rapid decline of IAP in the first 3 days after catheter drainage [40].
The inability to rehabilitate is significantly attributed to permanent intestinal injury that has been detected prematurely. Several authors advised a decompression procedure between a few hours and four days following the identification of abdominal compartment syndrome as shown in Table 2. Decompensated pulmonary or heart failure necessitates rapid decompression using surgery because of the dire outcomes and lack of other therapies (Table 2) [41-43].
| First author (year) | Severe acute pain | Intra-abdominal pressure | IAH-male (%) | Acute compartment syndrome | Interventions | Interventional treatment of ACS % | Time to intervention | ACS mortality |
|---|---|---|---|---|---|---|---|---|
| Tao | 345 | 2 | 14(67%) | 21 | Midline laparotomy with Bogota bag (n=18) | 85.7 | 9-22 h | 33.30% |
| De waele | 44 | 21 | 15(71%) | 4 | Midline laparotomy, temporary abdominal closure system (n=4) | 100% | - | 75% |
| Chen | 74 | 44 | 23(52%) | 20 | Percutaneous abdominal decompression and drainage (n=8); decompressive emergency laparotomy (n=8) | 65% | 26-33 h | 75% |
| Mentula | 26 | 0 | 23(88%) | 26 | Open abdomen (n=21) subcutaneous linea alba fasciotomy (n=5) | 100% | 1-5 days | 46% |
| Bezmarevic | 51 | 27 | 23(79%) | 6 | Midline laparotomy (n=6) | 83% | 1-4 days | 83% |
| Davis | 43 | 16 | 16(100%) | 16 | Midline laparotomy with Bogota bag (n=11) or wound Vacuum-Assisted Closure (VAC) system (n=5) | 100% | 3 h | 25% |
| Peng | 273 | 273 | 168(62%) | 273 | Midline laparotomy (n=61) | 23.30% | 2-101 h | 52.50% |
| Smit | 59 | 29 | 21(72%) | 13 | Transverse subcostal laparotomy (n=7), midline laparotomy (n=3) | 10(77%) | 1.9-15.5 days | 53% |
Table 2: Surgical management options for compartment syndrome of the abdominal wall in the backdrop of acute severe pancreatitis.
Data collection
Data were collected retrospectively from the computerized medical database for health records, including demographics, gender, Body Mass Index (BMI), length of hospitalization in an ICU, the number of ICU-free days (out of 60), period of residence again, fatality at the care facility, medical records, the disorder of pancreatitis and the type of hospitalization (original or referral) have been compiled. IAP laboratory investigations require supplementary therapy for kidney failure, quantity of solution given, fluid balance, quantity of fluid in the abdomen (degrees I, II and III according to amount; measured with CT), degree of daily measured Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation (APACHE) II score (days 1–14 in ICU).
Statistical analysis
Statistical Package for Social Sciences (SPSS) version 12.01, Chicago, IL, USA was used for statistical analysis. Spearman’s non-parametric relationship was used to analyze parameter interactions. The participants were divided into quartiles based on their highest IAP. The Kruskal-Wallis test was used to determine the statistical significance of the continuous variables across various categories.
Fisher’s exact test was used for the variable dichotomy. The Mann- Whitney U test was used to establish the significance of the two groups. The data presented is offered in the form of medians with Interquartile (IQ) ranges. The mean number of ICU-free days out of the 60 was recorded. Statistical importance was characterized by a p-value of ≤ 0.05.
The hospital mortality rate in the experimental group was 24% (18 out of 74) and the determination of IAH was 84% (62 out of 74). 34/74 (46%) of individuals had recurrences of IAH recurrence. ACS affected 36/74 (49%) patients, with recurrent ACS affecting 14/74 (19%). Table 3 outlines the demographic and physical information of survivors and non-survivors. The age of the participants was 46 years (range, 21-69 years) and gallstones were the major cause (84%). Ten patients underwent abdominal surgeries in the first two weeks in the ICU, followed by eight more. Four of the laparotomies performed throughout the study period were due to ACS. After surgery, in two cases, the IAP decreased from 34 mmHg to 17 mmHg-23 mmHg. The decline in IAP started within 24 h. The urine flow rate increased from 1 ml/kg/h to >2 ml/kg/h, lactate levels normalized and lung improvement occurred. After surgery, four individuals dropped their IAP from 25 mmHg to 13 mmHg. Urine flow rose from 0.5 randomized kg/h and lung improvement. In each of these individuals, the effects of IAP lasted several days. Intra-abdominal hemorrhage, attainable bowel perforation and proven or suspected peri-pancreatic necrosis infection were additional indications to consider laparotomy. 43% (32 of 74) of patients required substitute kidneys. The mortality rate was 18% in the 22 patients whose IAP was not assessed (Table 3).
| Characteristics | All (%) |
|---|---|
| Age | 54(100) |
| Male | 66(89) |
| Median age | 46(21-69) |
| Medium Body Mass Index (BMI) | 28(21-42) |
| History of comorbidity | |
| High blood pressure | 20(27) |
| Diabetes mellitus | 4(5) |
| Heart disorders | 6(8) |
| Hyperlipidemia | 4(5) |
| Chronic pancreatitis | 2(5) |
| Chronic Obstructive Pulmonary Disease (COPD) | 4(5) |
| Kidney | 4(5) |
| Psychological disorders | 8(11) |
| Cause | |
| Gallstone | 62(84) |
| Biliary | 12(16) |
| Fluid quantity by Computed Tomography (CT) | |
| Small | 26(35) |
| Moderate | 38(51) |
| Large | 8(11) |
| Balthazar degree | |
| A, B | 0(0) |
| C | 2(3) |
| D | 2(3) |
| E | 68(92) |
| no CT | 2(3) |
| Primary admission | 8(11) |
Table 3: Displays the clinical and demographic features of patients with SA.
Non-survivors had substantially higher max IAP, max APACHE II, max SOFA score, advanced age, peak lactate levels, top creatinine levels and baseline deficiency in ICU data as shown in Table 4 and minimal IAP had a substantial connection with a positive SOFA score (coefficient 0.49, p=0.0) and the relationship between peak Intra-Abdominal Pressure (IAP) and the maximum SOFA score in survivors and non-survivors of SAP (Figure 2 and Table 4).
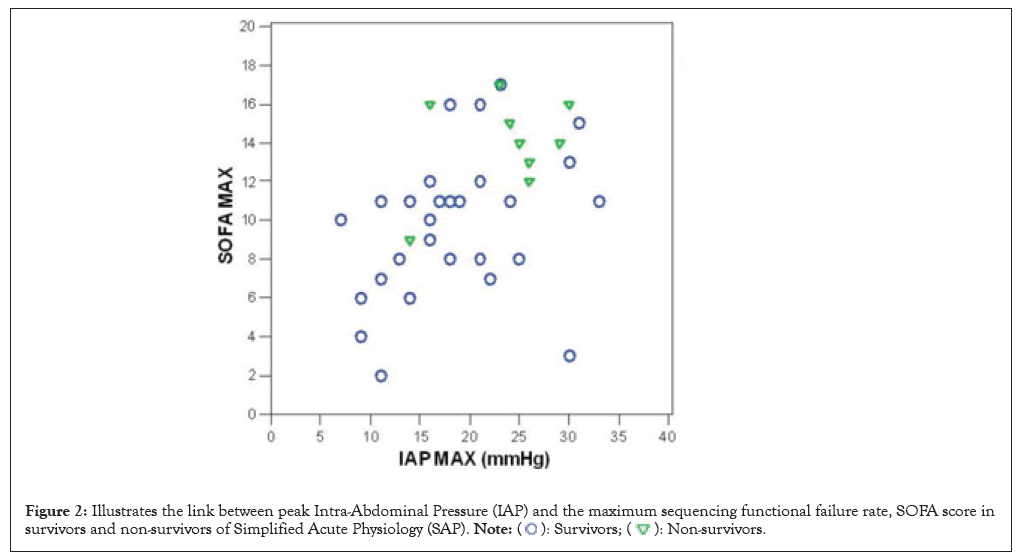
Figure 2: Illustrates the link between peak Intra-Abdominal Pressure (IAP) and the maximum sequencing functional failure rate, SOFA score in
survivors and non-survivors of Simplified Acute Physiology (SAP). 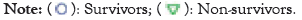
| Categories | Non-survivors | Survivors | p-value |
|---|---|---|---|
| Median (IQ range) | Median (IQ range) | ||
| IAP peak (mmHg) | 25(19.4-27.4) | 18(13.3-22.8) | 0.043 |
| SOFA max | 14(12.6-16) | 10.5(7.3-11.8) | 0.003 |
| ICU stay (days) | 27(7.10-54.10) | 15.5(7.3-20.8) | 0.257 |
| Hospital stay (days) | 28(9.15-107.15) | 26(20.0-37.5) | 0.986 |
| CRP at admission (mg/l) | 293(211-384) | 316(246-377) | 0.671 |
| APACHE II | 19(17.0-22.5) | 13(10.0-17.0) | 0.001 |
| Lactate max (mmol/l) | 2.7(2.1-7.1) | 1.5(1.3-2.1) | 0.006 |
| BE min (mmol/l) | -10.5(-13.2-(-8.0)) | -1.3(-6.6-(-1.3)) | <0.001 |
| Creatinine max (mmol/l) | 338(181.5-546) | 140.5(67.5-280.13) | 0.02 |
Note: IQ: Interquartile; IAP: Intra-Abdominal Pressure; SOFA: Sequential Organ Failure Assessment; ICU: Intensive Care Unit; CRP: C-Reactive Protein; APACHE: Acute Physiology and Chronic Health Evaluation; BE: Barium Enema.
Table 4: Shows ICU data from survivors and non-survivors of Simplified Acute Physiology (SAP).
On ICU days 1-7, non-survivors had the peak IAP, APACHE II, at hospitalization. The highest SOFA score and maximum were lactate, the highest creatinine level and base deficiency were substantially higher in non-survivors; there was a strong association of the highest IAP with the optimum SOFA score (the coefficient 0.49, p=0.001) as shown in Figure 3.
Figure 3: ICU, Maximal intra-abdominal pressure trial organ failure assessment and renal Sequential Organ Failure Assessment (SOFA) scores (1-7 days).
There was no notable appearance of SOFA scores in lung disease, kidney function, hospitalization or coagulation disorders (no data evidence was provided). It showed that non-survivors had higher total IAP, overall SOFA, SOFA cardiovascular and renal scores on ICU days 1-7 than survivors. Cases were divided into quartiles (18-20 cases per group) according to their highest IAP evaluation during days 1-14 in the ICU, with the highest IAP values ranging from 7 to 14, 16-18, 19-24 and 25-33 mmHg in categories 1-4. The overall mortality rates in groups 1-4 were 10%, 12.5%, 22.2% and 50%, respectively. The highest SOFA score (p=0.01), creatinine level (p=0.01), time spent in critical care (p=0.038) and lactate values (p=0.039) were also extremely significant (Kruskal-Wallis test). The variation in the mortality rates (1 and 4) was not deemed significant (p=0.14; Fisher’s exact test). In groups 1-4, the mean ICU-free days were 45.7, 38.8, 32.0 and 27.5 days, respectively (p=0.045, Kruskal-Wallis test). The distinction in ICU-free days between groups 1 and 4 was significant (p=0.023, Mann-Whitney test) (a p-value of 0.05 or less characterizes statistical importance.) The receiver operating characteristic curves for IAP max days 1-7, SOFA day 1 and APACHE II are shown in Figure 4.
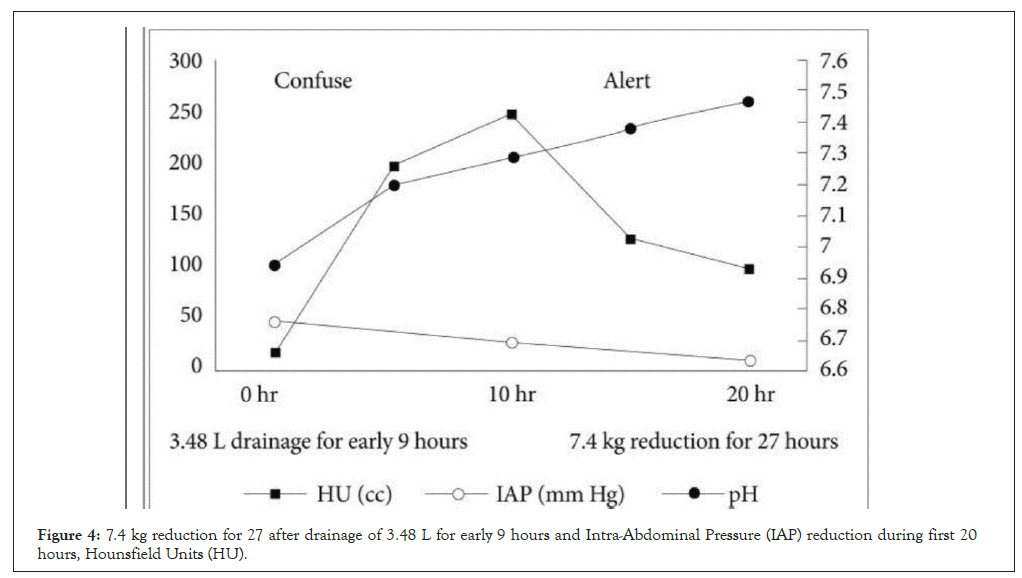
Figure 4: 7.4 kg reduction for 27 after drainage of 3.48 L for early 9 hours and Intra-Abdominal Pressure (IAP) reduction during first 20 hours, Hounsfield Units (HU).
Receiver Operating Characteristic (ROC) curves for IAP max days on the day of surgery were raised to 40-45 mmHg after the start of the disease. 7.4 kg reduction for 27 hours after drainage of 3.48 L for early 9 hours and IAP reduction during first 20 hours. Comatose patient became alert after the procedures as shown under ROC curve (Figures 3 and 4).
In the present investigation, researchers discovered that high IAP was correlated with organ failure and an extended stay in intensive care units in extremely sick patients with SAP. IAP augmentation is hazardous to all organs. Reduced cardiac activity, ventricular volume at end-diastolic preload, venous return and venous dilatation. Absence of breath is triggered by diaphragm elevation, which causes a decline in all respiratory parameters and a ventilation-perfusion mismatch that is normalized and important for artificial ventilation.
Renal dysfunction is associated with the decline of perfused blood because of the pooling of the peripheral blood caused by capillary and venous dilation; also, the cause of cardiac failure is decreasing venous return also by venous dilation and failure to output; the most likely explanation for the splanchnic infarction is vasopressin releasing. Systematic evaluation of severely deteriorated persons by classifications such as APACHE II and SAPS has been used [44]. Table 5 displays the leading factors for increased fluid collection due to highly dilated capillaries (Table 5).
| Mechanism | Eventually, Present in the AP |
|---|---|
| Determinants reducing abdominal wall compliance | Pain |
| Edoema of the abdominal wall | |
| Obese | |
| Instances that rose abdominal contents | Gastroparesis Ileus Ascites |
| Edoema with retroperitoneal hemorrhage | |
| Acute fluid accumulations | |
| DT hatminants that increase capillary permeability and fluid leake | Massive overloaded fluid |
| SIRS/MODS |
Note: (SIRS): Systemic Inflammatory Response Syndrome; (MODS): Multiple Organ Dysfunction Syndrome; (AP): Acute Pancreatitis.
Table 5: Factors affecting capillary leakage and excessive fluid collection.
The most common trigger cause of AP is alcoholic beverages (25% in Europe); however, in our study, the most common cause of AP was gallstone pancreatitis (84%).
Previous studies have related an increase in IAP to an increase in mortality in postoperative and trauma patients. Acute ACS has been linked to multi-organ malfunction and increased mortality in trauma patients and individuals with liver transplants. In a recent multicenter study, the mean ICU-free days declined considerably as the peak IAP increased in the quartiles stratified by maximal IAP. Recently, IAP was assessed when IAH was anticipated in patients with SAP and the frequency of IAH was 78%. This is supported by our finding that 84% of the participants had IAH. In the present study, non-survivors maintained their IAP throughout the first week in the ICU, whereas survivors IAP diminished [45]. The SOFA scores for non-survivors remained virtually unchanged. This could imply that IAP in patients with SAP is a significant indicator of poor outcomes. However, a more comprehensive inspection must be conducted to confirm this result.
The Sequential Organ Failure Assessment (SOFA) score is the degree of organ dysfunction; depending on the measure of the IAP score, the patients were divided into quartiles.
According to those characters, the chance of IAH in SAP is greater than 60%, whereas the prevalence of ACS in patients admitted to a critical-care unit ranges from 10% to 30%.
IAP was not tracked for all SAP patients in our ICU during the inquiry term, which represented oversight. Moreover, there was a limited patient population to explore the predictive impact of several variables on clinical results. However, the negative effects of high IAP on various organ systems have been demonstrated and IAH may be an obstacle to poor organ performance (SOFA score) in patients with SAP [46].
A combination of widespread splanchnic inadequate perfusion, restricted blood supply to the pancreas and bacterial translocation results in subsequent necrotizing pancreatitis with poor results. While the function and therapy of IAH in SAP are uncertain, IAH and CS are not explicitly addressed in the latest international guidelines for managing severe acute pancreatitis.
Although the primary data come from sustained IAH, especially ACS, has been linked to mortality.
A midline procedure is typically necessary for decompression with surgery in severe ill patients who not responding to medical therapy [47]. Regardless of the basic reason, decompressive laparotomy is particularly helpful in lowering the IAP in patients with ACS and this is another benefit observed in SAP. However, the significance of decompressive laparotomy remains controversial.
Leppaniemi, et al., [35] made tiny skin cuts in the front abdominal cavity; despite helping many people avert middle laparotomies, the method has drawbacks, including the complication of incisional hernias. In open laparotomies, facial sealing frequencies are boosted by enhanced by perfect sealing procedures. A few operators may favor a transverse cut in cases of SAP to allow further access to the pancreas.
The exact time of surgery for decompression is a fascinating subject. A study on a group of people receiving decompression therapy in Finland found a 100% mortality rate among individuals who underwent the procedure more than five days after the onset of their symptoms. It should not be surprising that organ dysfunction is permanently damaged in situations with extended exposure to high IAP [48]. However, it is challenging to identify the precise time window within which decompressive laparotomy can be effective. Early intervention, as early as 6 h following the onset of ACS, was found to be more beneficial by Ke, et al., [23] in animal research. Recent research proves that low-grade IAH is a less dangerous consequence than high-grade III-IV IAH and ACS, supporting the studies that earlier identification and handling of IAH contributes to easy management and improved outcomes.
When ACS has been identified, early therapy with surgical decompression is the best procedure; however, the actual evidence, threshold IAP values and other main approaches require further research. This is even more critical given the procedure’s high morbidity, especially when fascial sealing is difficult. This leaves an open abdomen with severe permanent complications and an urgent need for abdominal wall revision surgery. As established in traumatizing persons and other interventions, prompt decompressive laparotomy may minimize the risk of organ dysfunction in patients with serious IAH who have not responded to non-operative therapy.
In our study, prostigmine was used in medical trials in ICU critically ill patients, but restrictions, in pregnancy and breastfeeding will disqualify participation. When both of the following three symptoms are present, AP is diagnosed by standard abdominal pain, which is defined as a sudden appearance of a persistent, painful epigastric area that frequently radiates to the back; serum amylase or lipid activity is more than three levels above the limits of routine and AP is detected on transabdominal ultrasound, contrast-enhanced CT or MRI. Different strategies that contrast traditional treatment alone with traditional therapy is acceptable [49]. The standard course of treatment entails digestive decompression if required, painkillers, dietary encouragement, symptomatic relief, early revived fluids for IAH and elements of Chinese medicine, such as rhubarb and Glauber’s salt.
The term AP refers to the overall and local factors that contribute to an elevated risk of IAH in severe situations. The influence on prognosis correlates with a boost in morbidity involving renal, pulmonary and cardiovascular dysfunction, as well as mortality, especially among patients with ACS. IAH may have local consequences such as necrotizing infections. This process has been widely used.
The fluid regulation might be the most beneficial therapeutic strategy as a part of conservative therapy, which should be encouraging in all circumstances. Refractory ACS, on the whole, could require surgical decompression because of higher mortality if not promptly focused. Given that the typical incidence of ACS is fatal, this procedure will always be challenging and may be influenced by different experiences. Advances in urgent care, the rationale of procedure diagnosis, early enteral nutrition, alert fluid use and higher involvement in noninvasive treatments have all benefited the prognosis of AP, with overall death and morbidity rates reducing.
IAH is virtually non-existent in early acute pancreatitis, which is prevalent in patients with SAP. IAH is a phenomenon that has a harmful clinical impact while also being a factor that may reveal a poor prognosis. As a result, IAH monitoring should be recognized regularly in the context of SAP and attempts should be made to start early therapy to prevent the rise and advancement to ACS. In patients with chronic pancreatitis, frequent IAP assessment during acute intensive care is crucial for controlling abdominal perfusion pressure while recognizing individuals who might gain an advantage from early decompressive laparotomy.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Saad HA, Eraky ME, El-Tahe AK, Riad M, Farid MI, Sharaf K (2023) Compartmental Intra-Abdominal Pressure Risk in Severe Acute Pancreatitis is Prospectively Assessed. Trans Med. 13:310.
Received: 20-Nov-2023, Manuscript No. TMCR-23-28086; Editor assigned: 22-Nov-2023, Pre QC No. TMCR-23-28086 (PQ); Reviewed: 06-Dec-2023, QC No. TMCR-23-28086; Revised: 13-Dec-2023, Manuscript No. TMCR-23-28086 (R); Published: 21-Dec-2023 , DOI: 10.35248/2161-1025.23.13.310
Copyright: © 2023 Saad HA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.