Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research - (2021)
Objective: To compare tissue thickness over a Glaucoma Drainage Device (GDD) tube in patients with a pericardium graft versus scleral tunnel surgical technique.
Methods: A single center, retrospective case series was performed of thirteen patients (15 eyes; two patients with bilateral procedures). Those included underwent GDD surgery between January 2014 and December 2020 and had two Anterior Segment Optical Coherent Tomography (AS-OCT) images available in their patient chart. Fourteen eyes had an Ahmed® valve placement; one had a Baerveldt® 350. AS-OCT was used to measure tissue thickness above the GDD tube. The patients were subdivided into 2 groups: Group A (scleral tunnel for tube placement) and Group B (pericardium patch above GDD tube). The tissue thickness was compared to see if there were changes from the patient’s first available OCT after surgery, to the second OCT in the patient chart. Tissue thickness differences were also compared between the 2 groups.
Results: Group A initial tissue thickness above the GDD tube was 0.288 ± 0.102 millimeter (mm) and subsequently 0.252 ± 0.111 mm. Mean difference from the first OCT to the second was 0.036 mm. Group B initial tissue thickness above the GDD tube was 0.357 ± 0.0668 mm, and subsequently 0.253 ± 0.0879 mm. Mean difference of the values was 0.104 mm. The comparison between these means results in a t-value of 1.418 with a p value of 0.180.
Conclusion: There was no statistically significant difference in mean tissue thickness above a GDD tube with a pericardium graft when compared to a scleral tunnel. There was a statistically significant difference of mean tissue thickness reduction over time in the pericardium patch group B. No statistical difference was found in tissue thickness reduction in group A.
Glaucoma drainage devices; Pericardium grafts; Scleral tunnel; Anterior segment OCT; Glaucoma; Glaucoma surgery; Tube erosion
Glaucoma drainage devices (GDD) are an effective strategy in the management of glaucoma, they are however associated with significant risks including erosion of the tube with subsequent infection. To avoid erosion, the GDD tubes may be placed with grafts of different materials or may be placed inside a scleral tunnel [1]. The value of grafts has been put into question due to their high costs, cosmetic issues, risk of infection and rejection, and possible erosion [2].
In this study we present the differences over time in tissue thickness above the glaucoma valve tube in patients with scleral tunnel compared to those with pericardium grafts.
Glaucoma is a neurodegenerative disease of the eye and one of the leading causes of blindness in the United States. Increased Intraocular Pressure (IOP) leads to Retinal Ganglion Cell (RGC) death along with eventual optic nerve atrophy, leading to vision loss. At the moment reducing the IOP is the only effective strategy to stop the progression of the disease. IOP management includes pharmacological agents, laser procedures and surgery [3]. Surgical management is reserved for patients whose IOPs cannot be lowered sufficiently with pharmacotherapy or laser procedures. Glaucoma Drainage Devices (GDD) have been gaining more popularity in recent years and are favored options in cases in which a trabeculectomy has failed or is likely to fail. GDD’s can also be used as primary procedures, in the 5-year follow up of the Tube Versus Trabeculectomy study it was shown that GDD’s (Baerveldt® 350) had a higher success rate compared to trabeculectomy in eyes with prior intraocular surgeries [4]. GDD’s are also indicated for cases with active uveitis, neovascular glaucoma, inadequate conjunctiva and chronic contact lens use. These devices have several risks associated which include immediate post-surgery hypotony with non-valved GDDs, fibrous encapsulation of the GDD plate, infection, strabismus, corneal decompensation, tube obstruction and tube erosion leading to late onset endophthalmitis [5]. GDDs consist of a tube which is inserted into the anterior chamber, sulcus, or pars plana, depending on the scenario, and is connected to a valve which is itself secured at the episclera. Surgeons may place a graft, usually of donor pericardium, over the anterior part of the GDD’s tube, since it is thought it can reduce the risk of erosion of the tube through the overlying conjunctiva and its associated risk of infection [6]. Lankaranain et al. found that the rate of erosion with a single pericardium patch was of 16% and that placing a double graft did not erode [2,7]. Gdih Gdih and Kailun Jiang found that graft-free technique, using a scleral tunnel; decreased costs by 39%-45%, excluding valve costs and the rates of conjunctival and scleral erosion at two years were 2.4% and 0% respectively [8]. Other studies have shown that pericardium grafts may melt over time leading to erosion, wound leak and endophthalmitis [6]. Moreover, grafts can greatly increase costs of surgery [2,7]. In Puerto Rico, place of practice of the authors, pericardium graft costs can range from $250 to $300 in addition to the cost of the GDD itself, which greatly increases cost burden for patients. In the Commonwealth of Puerto Rico, 43.5% of its inhabitants live at a poverty rate. Therefore, economic factors play a significant role in determining the medical procedures to be practiced on patients. For these reasons, glaucoma valve grafts versus no grafts continue to be a controversial topic. Several studies have suggested that there might not be a true benefit to using graft patches over GDD tubes since other techniques can be as effective at preventing erosion, are less expensive and do not convey the inherent risks of using donor tissue, such as rejection and inflammation [9,10]. Studies performed to evaluate the efficacy of scleral tunnel technique versus pericardial graft technique in GDD surgeries have shown less tube exposure rate in the former [11].
A single center, retrospective chart review was conducted on patients who underwent GDD implantation, with either pericardium patch or scleral tunnel approach, between the years of 2014 and 2020. The reason for choosing one surgical method over the other was surgeon preference and patient economic considerations. In our institution pericardium grafts are not covered by medical insurance companies, therefore are paid out- of-pocket by the patient. Economic status of the patient is taken into consideration when the surgeon decides the surgical approach of GDD. Inclusion criteria were patients who underwent GDD and had two routine post-operative Anterior Segment OCT (AS- OCT) measurements in the patient chart. The first OCT at an average of 23.9 ± 19.7 months post-surgery and the second at an average of 16.13 ± 7.9 months after the first OCT. Exclusion criteria were: patients who did not undergo OCT measurements and those with only one imaging study. This study was ethically conducted in accordance with the Declaration of Helsinki.
OCT SPECTRALIS Tracking Laser Tomography® (Heidelberg Engineering, MA, USA) was used for the AS-OCT measurements. Thirteen patients were examined for a total of 15 eyes (6 right eyes and 9 left eyes); 2 patients underwent bilateral procedures. Subjects were divided into 2 groups. Group A consisted of patients in which 22 mm needle scleral tunnel with a minimum of a 5 mm tract and without graft patch was performed. Group B consisted of patients in which pericardium graft was used to cover the anterior portion of GDD tube. Two OCTs at different moments in time were used to compare tissue changes within each group. Due to this study being of retrospective nature, the time from surgery to first OCT measurements were not standardized. Table 1 shows the timeline of OCT imaging. A proportion of the known diameter of the valve tubes (Ahmed® (New World Medical, CA, USA) diameter=0.635 mm [12] Baerveldt 35® (Johnson & Johnson, FA, USA) diameter=0.630 mm [13] and the measured diameter of the tissue thickness above the tube was calculated using two different points along the tube’s course for each study. Paired and unpaired t-tests were performed to determine statistical significance in differences in tissue thickness among each group and between groups respectively.
| Year of surgery | Time from surgery to first OCT (months) | Time from first to second OCT (months) |
|---|---|---|
| Group A | ||
| 2017 | 20 | 21 |
| 2017 | 24 | 23 |
| 2017 | 46 | 5 |
| 2018 | 12 | 23 |
| 2018 | 14 | 22 |
| 2019 | 14 | 6 |
| Group B | ||
| 2014 | 84 | 6 |
| 2017 | 20 | 22 |
| 2017 | 27 | 22 |
| 2017 | 35 | 6 |
| 2018 | 10 | 15 |
| 2018 | 14 | 22 |
| 2018 | 9 | 22 |
| 2018 | 24 | 5 |
| 2019 | 6 | 22 |
Note: OCT: Optical Coherence Tomography; GDD: Glaucoma Drainage Device
Table 1: Timeline of OCT measurements.
The mean ages for group A and group B were 62.17 ± 12.45 and 67.75 ± 9.38 years, respectively. Group A had an initial measurement of the mean tissue thickness above the GDD tube of 0.288 ± 0.102 mm and it was subsequently 0.252 ± 0.111 mm. Figure 1 shows changes in mean tissue thickness above GDD for Group A. The difference between these two means was 0.036 mm with a standard error of the difference of 0.019, confidence interval from -0.0134 to 0.0857 and a non-statistically significant p value of 0.120. Group B had an initial mean tissue thickness above the GDD tube of 0.357 ± 0.0668 mm, and it was subsequently 0.253 ± 0.0879 mm. Figure 2 shows mean tissue thickness above GDD for group B. The difference between these two means was 0.104 mm with a standard error of difference of 0.038, confidence interval from 0.0161 to 0.192 and statistically significant p value of 0.0259.
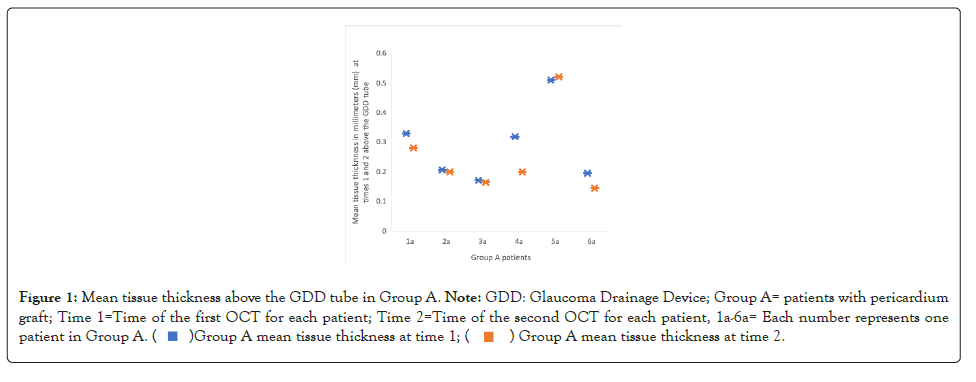
Figure 1:Mean tissue thickness above the GDD tube in Group A. Note: GDD: Glaucoma Drainage Device; Group A= patients with pericardium
graft; Time 1=Time of the first OCT for each patient; Time 2=Time of the second OCT for each patient, 1a-6a= Each number represents one
patient in Group A.
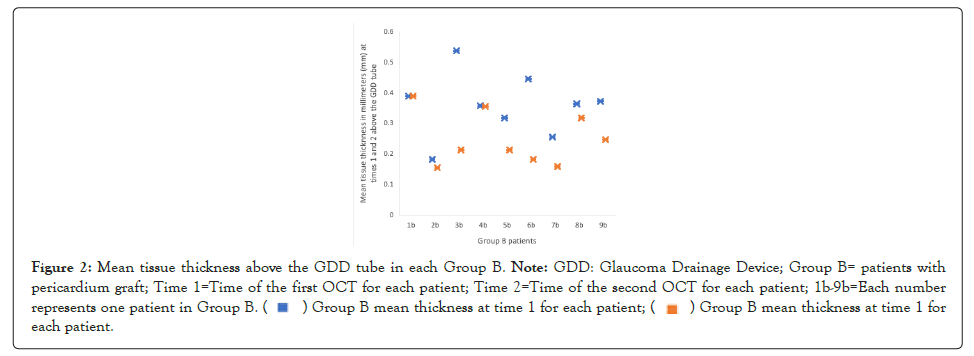
Figure 2:Mean tissue thickness above the GDD tube in each Group B. Note: GDD: Glaucoma Drainage Device; Group B= patients with
pericardium graft; Time 1=Time of the first OCT for each patient; Time 2=Time of the second OCT for each patient; 1b-9b=Each number
represents one patient in Group B. Group B mean thickness at time 1 for each patient.
Group B mean thickness at time 1 for each patient.
Table 2 displays the mean of the thickness above the tube for the two images within each group. For group A the mean difference from the first Optical Coherence Tomography (OCT) to the second was 0.0398 mm. For group B the mean difference of the values was 0.110 mm. The comparison between these means results in a t-value of 1.418 with a p-value of 0.180 demonstrates no statistically significant difference (p>0.05) between the changes in mean tissue thickness in the OCT’s between groups A and B. No GDD tube (0%) exhibited erosion throughout our study period.
| Group A 1st OCT | Group A 2nd OCT | Group B 1st OCT | Group B 2nd OCT |
|---|---|---|---|
| 0.33 | 0.28 | 0.388 | 0.388 |
| 0.206 | 0.2 | 0.182 | 0.154 |
| 0.17 | 0.165 | 0.536 | 0.212 |
| 0.318 | 0.2 | 0.357 | 0.355 |
| 0.509 | 0.52 | 0.318 | 0.212 |
| 0.194 | 0.145 | 0.445 | 0.182 |
| 0.254 | 0.159 | ||
| 0.363 | 0.318 | ||
| 0.371 | 0.246 |
Note: OCT: Optical Coherence Tomography; GDD: Glaucoma Drainage Device; Group A=Patients with scleral tunnel; Group B=Patients with pericardium graft
Table 2: Mean tissue thickness in the first and second OCT in millimeters (mm) above GDD tube for patients in group A and group B.
We set out to measure tissue thickness differences of 2 types of surgical procedures used for GDD. We found there was no statistically significant difference in tissue thickness above the GDD tube in patients with scleral tunnel compared to those with grafts, indicating there might not be an additional benefit to the use of grafts. If this is indeed true, choosing the latter could help us reduce the costs of GDD surgery while maintaining the best standard of care for our patients. Interestingly, to the authors of this study, a statistically significant difference in tissue thickness above GDD tube was found for patients with a pericardium graft over the GDD tube, however, the same was not true for patients with a scleral tunnel. Nevertheless, a larger sample size and longer follow up periods would be required to confirm whether these results are upheld. We had 0% tube erosions in either group during the time of the study. As with all retrospective studies, we recognize some of our limitations include selection bias and since these patients were not intended for a clinical study our OCT measurements were not standardized in time, which could affect our results. Other limitations include its small sample size [14].
Additionally, we only studied patients with either a pericardium graft or scleral tunnel, yet other types of grafts can also be used and have been shown in previous studies to have decreased rates of exposure. To our knowledge, this is the first study to perform serial AS-OCT measurements to compare different tissue thickness above the GDD. Further studies are warranted to determine if tissue thickness is an indication of a risk factor for complications such as tube erosion and if grafting reduces these risks.
Citation: Ramos-Bartolomei S, Rivera-Grana E, Ulloa-Padilla J, Blasini-Torres B (2021) Comparison of Tissue Thickness over Glaucoma Drainage Device Tube with and Without Pericardium Graft. J Clin Exp Ophthalmol. 12:901
Received: 01-Dec-2021 Accepted: 15-Dec-2021 Published: 22-Dec-2021
Copyright: © 2021 Ramos-Bartolomei S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.