Clinical Pediatrics: Open Access
Open Access
ISSN: 2572-0775
ISSN: 2572-0775
Research Article - (2019)Volume 4, Issue 2
There exists evident differences in the health and the care received of the Amish people in comparison to the general population. Amish patients were 100% homozygous for delta F508 mutation versus 12% in the non-Amish population (P=0.057). The Amish community was prescribed dietary supplements at a rate of 89% versus 50% for non-Amish (P=0.087). BMI > 75th percentile was 51.25% in Amish patients and 61.25% in non-Amish patients (P=0.31). Mean FEV1 value was 83.6% versus 100% while mean FEF 25-75 value was 58% versus 86% for Amish and nonAmish people respectively. There was substantial variance in the number of episodes of the same infection per patient between Amish and non-Amish population (1 IQR 0–1 versus 0 IQR 0–1; p=0.012). The rate of antibiotic prescribing per patient in the Amish group was 7.9 perceptions versus 2.2 prescriptions. The study aims to create the best possible treatment and the highest quality of life for patients with cystic fibrosis. Our manuscript creates a paradigm for future studies on the delta F508 mutation, nutrition supplements, body mass index (BMI), forced expiratory volume in 1 second (FEV1), forced expiratory flow (FEF 25-75), airway microbial organisms and changes in antibiotic treatments in Amish compared to the non-Amish group, as well as developing a platform for an Amish community health program.
Delta mutation outcomes; Nutritional status; Lung function; Antibiotic therapeutic; Amish; Non-Amish Pediatric; Cystic fibrosis
Cystic Fibrosis (CF) is a progressive, hereditary illness that causes persistent lung infections and curbs the ability to breathe over time [1]. Despite the same genetic mutations, the disease expression is variable and depends on environmental, cultural, behavioral, psychosocial, and economic background [2-5]. Pulmonary disease is the most common cause of morbidity and mortality in patients with CF [6]. Mucus secretion increases and traps bacteria causing infections, expanded lung damage, and finally, respiratory failure [1]. Haemophilus influenzae and Staphylococcus aureus usually reside below the mucus and can grow uncontrollably in infants and children with CF. Pseudomonas aeruginosa often affects patients throughout adulthood [7]. With the advent of new bacterial culture methods, it is evident that the airways of patients with CF are colonized recurrently with polymicrobial infections >75th percentile7]. Treating infections in CF is multifarious, comprising antibiotics, anti-inflammatory agents, chest physiotherapy, and inhaled medications to improve mucus clearance [8].
More than 30,000 people in the United States have CF, and about 1000 new cases are identified annually [1]. Sixty-five percent of CF patients are diagnosed as a newborn, and 75% of cases are detected by two years of age [9]. Half of the individuals with CF are 18 years of age or older [1,9]. High allele frequencies (1:20) are observed in a Caucasian population with a prevalence of 1 in 3,500 individuals [9-12].
The Amish community was a part of the Swiss-German migration to North America in the seventeenth century [10]. Their approximate population in North America is 318,390 [13]. They hold different lifestyle philosophies relative to health care believes, insurance, and religion [10,13]. They use traditional farming methods that rely on horses for field work and transportation [10,13].
Estimating the number of Amish affected with CF is challenging as this population is primarily understudied and underreported [14]. Previous research from Ohio in one Amish community reported that the occurrence of cystic fibrosis amongst the population was 1 in every 569 live births. There were no reported cases of cystic fibrosis in 4,448 live births in the second Amish community [13,15]. However, higher rates of CF exist amongst the Amish people compared to the general population [10,13].
The aim of this study was to explore the clinical manifestations and outcomes in the Amish community with CF regarding the delta F508 mutation, nutrition, Body Mass Index (BMI), forced expiratory volume in 1 second (FEV1), forced expiratory flow (FEF 25-75), patterns of airway microbial colonization and changes in antibiotic treatment regimen compared to the non-Amish community.
Patients and study design
Study protocol approval was obtained from Lutheran Hospital’s institutional review board before initiation. This single site, openlabeled, non-interventional, retrospective case-controlled study was conducted at a cystic fibrosis clinic at the Lutheran Children's Hospital. The clinic had a total of 40 CF patients and 25% of patients were Amish. This study included subjects aged 2 to 35 years of age with CF. Consent forms detailing the study purpose, procedure, risks, and benefits were signed before study enrollment by the patient. Participants were excluded from the study if they failed to give their informed approval. This comparative study of cystic fibrosis therapy in the Amish population was carried out and compared to the non-Amish community.
The primary outcomes investigated included homozygous for delta F508 mutation, nutrition, Body Mass Index (BMI), Forced Expiratory Volume in 1 second (FEV1), Forced Expiratory Flow (FEF 25-75), airway microbial organisms and changes in antibiotic treatments in Amish compared to the non-Amish group. Optimal antibiotic therapy was determined as antibiotic targeted to the offending pathogen and included de-escalation or discontinuation of unnecessary antimicrobial directed at other organisms. A contaminated culture was suspected if only 1 of the cultures was positive for commonly recognized contaminants such as “coagulase-negative Staphylococci”. The secondary outcome was to understand the impact of lifestyle factors in Amish population with CF including access to health insurance, utilizing electricity and physical activity level that may contribute to clinical result compared to the non-Amish group.
Data included age, homozygous for delta F508, height, weight, BMI, weight for length, FEV1, FEF 25-75, microbial colonization models, and antibiotic exposure [16]. Additional data obtained included: vaccine history, medications, treatment, insurance programs, assistance programs, power use, and well water [17,18]. Trained interviewers collected data every 3 months over a 5-year period [19]. A questionnaire related to non-port CF data was utilized to obtain supplementary information during one office visit and included access to financial assistance programs and electricity, and level of physical activity which was classified using Physical Activity Questionnaire (PAQ) [20].
Chi-square or Fisher's exact test was applied for an inferential statistic on categorical data, while Student's t-test with a 95% confidence interval was applied for continuous data. Wilcoxon test was applied to episodes of infections due to non-normal distribution.
This retrospective analysis included data from 25 CF patients: Amish (n=9) and non-Amish (n=16). There was no statistical difference in age and sex between the groups (Table 1). The Amish population with CF were 100% homozygous for delta F508, while 40% of the non-Amish group was heterozygous, 12% were homozygous, and 8% did not carry the delta F508 mutation (p=0.057). The Amish population was prescribed dietary supplements at a rate of 89% versus 50% for non-Amish (p=0.087). The prevalence of a BMI >75th percentile was 51.25% in Amish patients and 61.25% in non- Amish patients, with the variation not statically significant (p=0.31) (Figure1). The mean FEV1 % was 83.6% versus 102.5% (Figure 2) while the average FEF 25%-75% value was 58% versus 86% in Amish compared to the non-Amish group.
| Baseline Characters (n=25) | Amish Group (n=9) | Non-Amish Group (n=16) |
|---|---|---|
| Sex (% male) | 33% | 37.50% |
| Average Age | 7 | 7.75 |
| % with Insurance | 55% | 100% |
| % with Electricity* | 11%* | 100%* |
| % with Well Water* | 100%* | 31%* |
| % Given Oral Supplements | 88% | 50% |
* indicates statistical significant difference for numbers between Amish and non- Amish groups (p<0.05)
Table 1: Demographics of the Amish and Non-Amish groups.
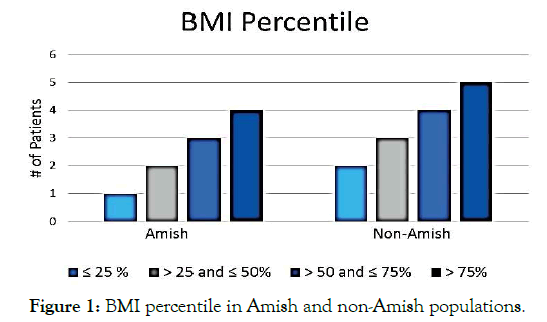
Figure 1. BMI percentile in Amish and non-Amish populations.
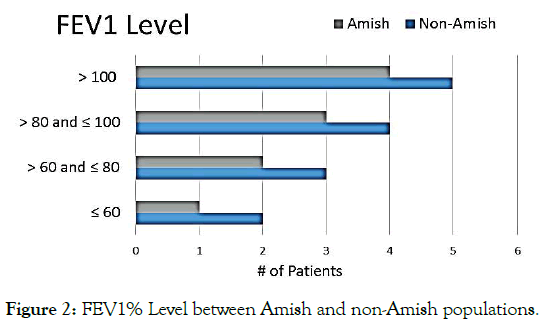
Figure 2. FEV1% Level between Amish and non-Amish populations.
Organisms identified in the sputum of patients with CF were Haemophilus influenzae, Staphylococcus aureus (MSSA), Methicillinresistant Staphylococcus aureus (MRSA), Haemophilus parainfluenzae (H. parainfluenzae), Pseudomonas aeruginosa (P. aeruginosa), Stenotrophomonas maltophilia, Salmonella heidelberg, Enterobacter cloacae complex, Acinetobacter ursingii, Klebsiella oxytoca, Acinetobacter lwoffi, Escherichia hermannii, Citrobacter freundii, Candida albicans, Bordetella bronchiseptica, Streptococcus pneumoniae, Pseudomonas putida, Acinetobacter baumannii complex, Candida lusitaniae, Escherichia coli, Enterococcus faecalis, Beta Hemolytic Streptococcus Group B, Candida parapsilosis, Candida rugose, Aspergillus fumigatus, Beta Hemolytic Streptococcus Group A, Klebsiella pneumoniae and Enterobacter cloacae. The mean number of total episodes of infection per patient in Amish population was 9 versus 8.3 in non-Amish people, with the most prevalent organism being H. parainfluenzae (90/417 episodes in Amish versus 134/476 in non-Amish) (p=0.672).
There was a considerable difference in the number of incidents of the same infection per patient between Amish and non-Amish population (1 IQR 0–1 versus 0 IQR 0–1; p=0.012). The average rate of antibiotic prescribing per patient in Amish group was 7.9 perceptions (95% CI, from -3.1 to 3.1) versus 2.2 prescriptions in non-Amish group (95% CI, from -1.1 to 1.1), which is the statistically significant difference (p=0.0035). Antibiotic usage most significant for both CF groups was tobramycin (30 out of 79 in Amish versus 6 out of 28 in non-Amish) (p=0.046). 40% of the Amish population used insurance programs and financial assistance program compared to 100% of the non-Amish population (Table 1). Fortyfour percent of the Amish group had electricity in the home compared to 100% in the non-Amish group (p=0.001, OR: 0.0053) (Table 1). The average of activity level in the Amish population was higher at 4.12 as compared to the non-Amish community at 3.4 (Figure 3). Use of well water in the Amish was 9 individuals versus 5 individuals for the non-Amish group (p=0.001, OR: 401/1). All patients were up to date on recommended vaccines.
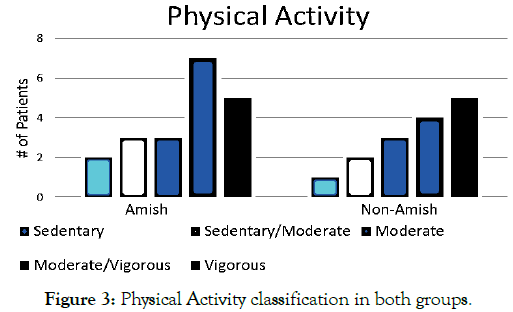
Figure 3. Physical Activity classification in both groups.
Although the number of subjects reviewed was small, there were significant differences and trends noted for many of the observation points. The Amish are more likely to be homozygous delta F508 as compared to the non-Amish population. The study also finds lower FEV1, FEF 25-75, and BMI. Higher physical activity levels were found in the Amish community compared to non-Amish community. Despite higher numbers of prescriptions for supplemental nutrition, the Amish group had lower BMI, which may be secondary to increased physical activity and increased resting energy expenditure which requires further exploration. The result of our study showed that there is a substantial difference in bacterial colonization and antibiotic usage between Amish and non-Amish. The outcomes are consistent with a recent study that has demonstrated an increased annual prevalence of H. parainfluenzae in CF patients [19]. Although H. parainfluenzae has been considered a prevalent isolate, we did not find any association between the presence of H. parainfluenzae and worsening of the lung function.
Minimization of the impact of infection and consequent pulmonary damage is crucial. The problematic issue in the treatment of cystic fibrosis disease is an emergence and spread of antibiotic resistance. The extensive use of aggressive antibiotic therapies in CF disease has produced an increased risk of developing antibiotic resistance, as confirmed in the pathogens MSSA and P. aeruginosa. According to cystic fibrosis pulmonary guidelines, 2013, CF patients infected with P. aeruginosa are managed by administering inhaled antibiotics in 28-day every month [21]. Tobramycin antibiotics are often used to treat infection due to P. aeruginosa, Serratia marcescens and Burkholderia gladioli in Amish population. However, tobramycin antibiotics are commonly used to treat infection with P. aeruginosa in the non-Amish community. Serratia marcescens and Burkholderia gladioli might be more frequent in Amish people due to their lifestyle which requires further search.
This study analyzed the utilization of services, access to care, and the corresponding clinical outcomes in the Amish compared to the non-Amish population. The research shows low access to financial services, electricity, and insurance programs in the Amish community. Although, they use substantially more well water, they take care of their children and bring them to the clinic during exacerbations. The study suggests that the differences in the Amish with cystic fibrosis and the non-Amish patients concerning lifestyle and environment can impact the outcomes of the care they receive. Adjustments in treatments to better serve the Amish population may be warranted. Further studies are needed to understand if antibiotic therapy for the significant pathogens and the treatment of infective exacerbations is the best approach in the presence of novel antibiotic drug formulations.
The authors declare no conflicts of interest. The authors thank Gordon Bokhart Pharm D, Alexis E. Schrieber, Pharm D, and Rachel McCafferty, Research Student.
Citation: Padmalingam RP, Gilham D, Laturner T, Jones R, Bokhart G, Hussien A, et al. (2019) Comparison Of Homozygous For Delta F508 Mutation Nutritional Status, Lung Function Outcomes, Bacterial Growth, Antibiotic Treatments And Therapeutic Outcomes Between The Amish And Non-Amish In A Single Pediatric Cystic Fibrosis Centre: A Retrospective Case-Controlled Study. Clin Pediatr OA 4:151
Received: 04-Jan-2019 Accepted: 19-Feb-2019 Published: 26-Feb-2019 , DOI: 10.35248/2572-0775.19.4.151
Copyright: Copyright© 2019 Padmalingam RP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited