Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2021)
Background: Acquired reactive perforating collagenosis (ARPC) is a skin disease characterized by multiple itchy nodules, which is notably similar to the skin eruptions of prurigo nodularis (PN). The aim of this study was to prove the clinicopathological differences between ARPC and PN.
Methods: We examined 22 patients with ARPC (6 males and 16 females) and 38 patients with PN (27 males and 11 females), diagnosed clinically and histologically. Transepidermal elimination of collagen which is characteristic histological findings for ARPC was found in all ARPC patients, but not in PN patients even in a serial section. Clinical findings, laboratory data, histological findings, immunohistochemical results, and efficacy of the therapies were compared between the two groups.
Results: On evaluation of laboratory data, thymus and activation-regulated chemokine (TARC) levels and peripheral eosinophil counts were lower in ARPC than in PN. Histologically, we observed greater infiltration of neutrophils, histiocytes, and lymphocytes and more extensive capillaries in ARPC than in PN. Upon immunohistochemical analysis, CD163+ macrophages were found to be more abundant in the upper-to-deep dermis of ARPC, and both CD4+ T cells and CD8+ T cells were significantly increased in the mid-to-deep dermis of ARPC. The efficacies of topical steroid and antihistamines were improved by the addition of emollients in ARPC.
Conclusion: This is the first report to show the histological differences in cell infiltration between the lesions of ARPC and PN. It is likely that inflammation mediated by neutrophils and M2 macrophages may be involved in the formation of ARPC lesions, as against PN lesions mediated by epidermal changes such as spongiosis. It is suggested that the addition of emollients can increase the efficacy of treatments for ARPC. However, further studies are necessary to define the precise pathomechanisms of ARPC and the effects of emollients on ARPC.
Acquired reactive perforating collagenosis, Prurigo nodularis, CD163, M2 macrophage, Neutrophil, Emollient, Transepidermal elimination
Acquired perforating dermatosis is a group of skin disorders in adults, histologically characterized by transepidermal elimination (TEE) of dermal tissue components. It includes acquired reactive perforating collagenosis (ARPC), perforating folliculitis, elastosis perforans serpiginosa, Kyrle’s disease, perforating calcifying elastosis, and perforating granuloma annulare [1]. Faver et al. [2] stated the diagnostic criteria of ARPC as follows: age of disease onset after 18 years, the presence of umbilicated papules or nodules with a central adherent plug, and the elimination of necrotic collagen tissue within an epithelium-lined crater. In addition, itch, scratching, and the presence of Koebner’s phenomenon are important as clinical characteristics [3].
Prurigo nodularis (PN) is also characterized by multiple itchy nodules in adults. As TEE is the most important point to distinguish between ARPC and PN, serial sections are often needed to find TEE. Thus, in this study, we aimed to find out other factors than TEE in the histopathology and laboratory data for better indicators to distinguish ARPC and PN.
The study was conducted in compliance with the ethical principles of the Declaration of Helsinki and was approved by the Ethics Committee of Gifu University Graduate School of Medicine (approval ID: 2020-047, 0345). This was a retrospective study on patients with ARPC and PN diagnosed between January, 2014 and December, 2019 at Gifu University Hospital.
TEE of collagen was found in ARPC (Figure 1), but not in PN even in a serial section. In total, 22 patients with ARPC (6 males and 16 females) and 38 PN (27 males and 11 females) were included in the study.
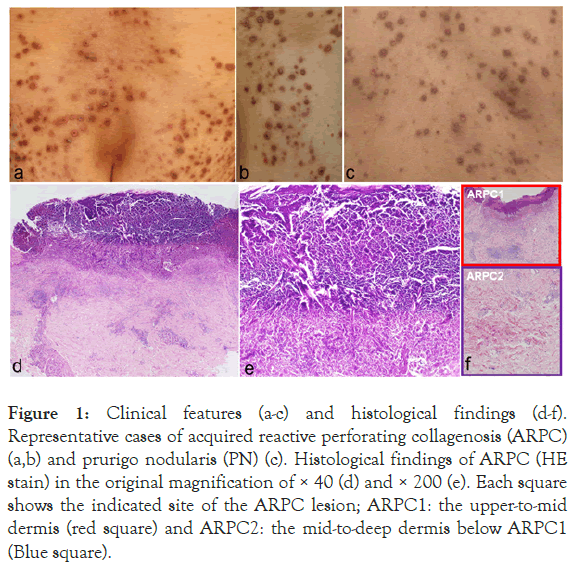
Figure 1: Clinical features (a-c) and histological findings (d-f). Representative cases of acquired reactive perforating collagenosis (ARPC) (a,b) and prurigo nodularis (PN) (c). Histological findings of ARPC (HE stain) in the original magnification of × 40 (d) and × 200 (e). Each square shows the indicated site of the ARPC lesion; ARPC1: the upper-to-mid dermis (red square) and ARPC2: the mid-to-deep dermis below ARPC1 (Blue square).
The main clinical findings of both diseases were the presence of multiple itchy nodules with crusts (Figure 1). The average age of the patients at the first consultation was not different between ARPC and PN. The severity scores for ARPC and PN were evaluated as the scores for perforating dermatoses [4]. The severity scores were divided into five levels: 0-1: near remission, 2-6: mild, 7-15: moderate, 16-33: severe, and 34-46: most severe. We also compared the time from onset to the first visit, subjective symptoms, the severity of skin eruptions, and comorbidities between the two groups. In addition, treatments and the efficacy were compared between the two groups. Efficacy was clinically evaluated by 4 grades; significantly improved, slightly improved, no change, and worse.
Laboratory data including leukocyte, eosinophil, neutrophil and basophil counts, and serum levels of creatinine, thymus and activation-regulated chemokine (TARC), IgE, IgG, IgA, IgM, aspartate transaminase (AST), and alanine aminotransferase (ALT) at the first visit were compared between the two groups.
We examined the histological findings in the dermis by HE stain; the upper-to-mid dermis at the nodule (ARPC1) and the midto- deep dermis below ARPC1 (ARPC2) in ARPC (Figure 1). In addition, the upper-to-mid dermis at the nodule (PN1) and the mid-to-deep dermis below PN1 (PN2) were examined in PN.
Lymphocytes, neutrophils, histiocytes, and eosinophils were counted in four different high-power (x400) fields (HPF). The average of four HPFs (0: none, 1: 1-9 cells/HPF, 2: 10-49 cells/HPF, 3: more than 50 cells/HPF) were used to evaluate the histological scores of infiltrated cells. In addition, spongiosis and increased capillaries were evaluated as the average of points in four HPFs: severe (2 points), mild (1 point) or absence (0 point).
Immunohistochemical staining was performed on 3 μm sections of formalin-fixed paraffin-embedded specimens using the standard method (the streptavidin-biotin-peroxidase technique). The sections were incubated with the primary monoclonal antibodies: anti-CD4 (clone SP35, Cell Marque), anti-CD8 (clone 4B11, Novocastra), anti-CD20 (clone L261.3.4, DAKO), anti-CD56 (clone 123C3.D5, Novus Biologicals), anti-CD163 (ab87099, Abcam), anti-CD68 (KP1, Abcam), anti-basophils (2D7, BioLegend), and transforming growth factor (TGF)-ß3 (R & D Systems). After overnight incubation with the primary antibodies, the sections were incubated with the Envision reagent (DAKO, Copenhagen, Denmark). Finally, antibody binding was visualized by incubating with the horseradish peroxidase substrate diaminobenzidine, and the sections were counterstained with hematoxylin. Cells that were positively stained for anti-CD4, -8, -20, -56, -163 and -68, and basophils were evaluated on an average of four HPFs.
Statistical comparisons of laboratory data and cell counts were performed using a two-tailed Student’s t-test and Mann–Whitney U test, and significant differences were defined at p<0.05.
Time from onset to the first visit was significantly shorter (p<0.05) in ARPC than in PN (Table 1). Subjective symptoms were mainly itch in both groups. Severity scores were severe in 4, moderate in 11, and mild in 7 patients with ARPC, and severe in 5, moderate in 20, and mild in 13 patients with PN (Table 1). No patients with near remission or most severe were observed in both groups. Frequencies of comorbidities were almost the same, and diabetes mellitus was the most frequent in both groups (Table 1).
| ARPC (n=22) | PN (n=38) | |
|---|---|---|
| Sex (patients) | Male 6: Female 16 | Male 27: Female 11 |
| Age (years) | 58.5 ± 16.7 (18-83) | 62.1 ± 15.2 (29- 84) |
| Time from onset to the first visit (months) | 5.85 ± 8.71* (0.25-36) | 22.1 ± 28.3* (0.5-108) |
| Subjective symptoms (patients) | Itch 21, Pain 1 | Itch 38 |
| Severity of skin eruptions (patients) | Severe: 4, Moderate: 11, Mild: 7 | Severe: 5, Moderate: 20, Mild: 13 |
| Comorbidity (patients) | 19 (86.4%) | 27 (71.1%) |
| Diabetes mellitus: 8 (36.4 %) | Diabetes mellitus: 8 (21.1%) | |
| Internal malignancy: 5 (22.7%) | Hypertension: 8 (21.1%) | |
| Autoimmune diseases: 3 (13.6%) | Hyperlipidemia: 4 (10.5%) | |
| Atopic dermatitis: 2 (9.1%) | Hyperuricemia: 2 (5.3%) | |
| Hyperlipidemia: 2 (9.1%) | Hepatitis C: 2 (5.3%) | |
| Hyperuricemia: 2 (9.1%) | Internal malignancy: 1 (2.6%) | |
| Renal failure: 2 (9.1%) | Hepatitis B: 1 (2.6%) |
*p<0.05
Table 1: Clinical characteristics of acquired reactive perforating collagenosis (ARPC) and prurigo nodularis (PN).
Combination of topical steroid and antihistamines were effective (significantly improved and slightly improved) in both groups. Addition of emollients such as white petrolatum and heparinoid cream significantly increased the efficacy in ARPC than in PN (Table 2). Other therapies such as narrow-band ultraviolet B (UVB) therapy, diaphenylsulfone, minocycline, cyclosporine and prednisolone were given to both groups (Table 2).
| Treatment | Efficacy 1) (%) | |
|---|---|---|
| ARPC | PN | |
| Topical steroid+emollient+antihistamine | 8/13 (61.5)* | 5/31 (16.1)* |
| Topical steroid+antihistamine | 2/7 (28.6) | 1/5 (20) |
| Topical steroid | 1/1 (100) | 1/2 (50) |
| Narrow band UVB therapy | 5/5 (100) | 11/14 (78.6) |
| Diaphenylsulfone | 1/1 (100) | 1/4 (25) |
| Minocycline | 1/2 (50) | 6/7 (85.7) |
| Cyclosporine | 0/1 (0) | 3/5 (60) |
| Prednisolone | - | 3/4 (75) |
*p<0.05
1) The number of the patients showing efficacy (significantly improved or slightly improved)/the number of the patients treated.
Table 2: Efficacy of treatments on acquired reactive perforating collagenosis (ARPC) and prurigo nodularis (PN).
Peripheral eosinophil counts and TARC levels were lower in ARPC than in PN (Figure 2). Eosinophilia was observed in 5 ARPC patients (22.7%) and in 14 PN patients (36.8%). IgE levels were elevated in 15 out of 23 PN patients tested (65.2%), and in 7 out of 11 ARPC patients tested (63.6%). TARC levels were elevated in all 15 PN patients tested (100%), and in 6 out of 10 ARPC patients tested (60%). Leukocyte, neutrophil, and basophil counts, and serum levels of IgE, IgG, IgA, IgM, AST, and ALT were not significantly different between the two groups (Figure 2).
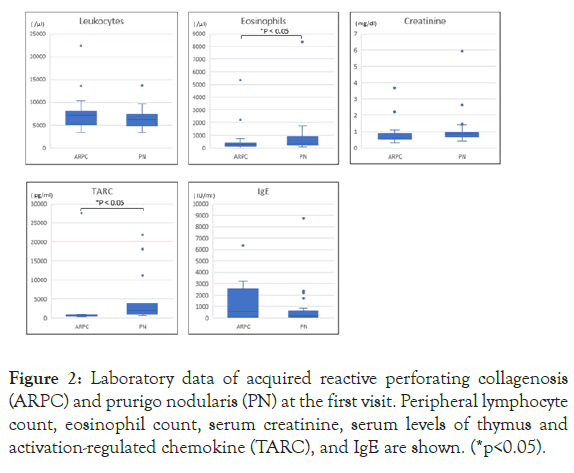
Figure 2: Laboratory data of acquired reactive perforating collagenosis (ARPC) and prurigo nodularis (PN) at the first visit. Peripheral lymphocyte count, eosinophil count, serum creatinine, serum levels of thymus and activation-regulated chemokine (TARC), and IgE are shown. (*p<0.05).
Greater neutrophil and histiocyte infiltration was observed in the upper-to-mid and mid-to-deep dermis of ARPC compared to PN (Figure 3). Spongiosis was significantly more observed in the epidermis of PN than in that of ARPC. Greater infiltration of lymphocytes and more capillaries were seen in the mid-to-deep dermis of ARPC2 (Figure 3).
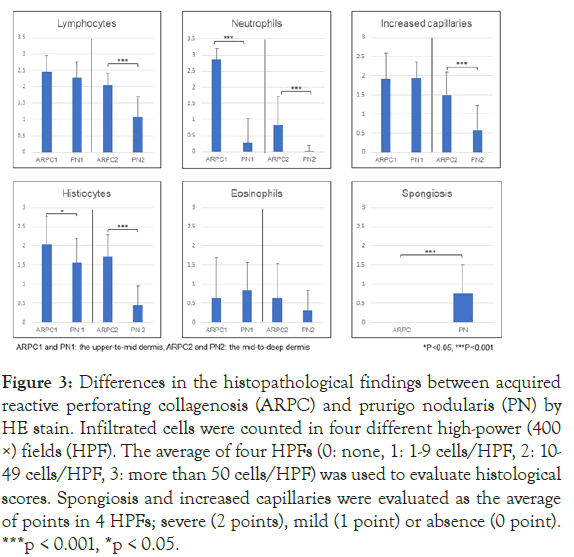
Figure 3: Differences in the histopathological findings between acquired reactive perforating collagenosis (ARPC) and prurigo nodularis (PN) by HE stain. Infiltrated cells were counted in four different high-power (400 ×) fields (HPF). The average of four HPFs (0: none, 1: 1-9 cells/HPF, 2: 10- 49 cells/HPF, 3: more than 50 cells/HPF) was used to evaluate histological scores. Spongiosis and increased capillaries were evaluated as the average of points in 4 HPFs; severe (2 points), mild (1 point) or absence (0 point). ***p < 0.001, *p < 0.05.
Eosinophil infiltration in the dermis was found in 4 out of 5 ARPC patients with eosinophilia (80%), and in 11 out of 14 PN patients with eosinophilia (78.6%). It was not statistically different between the two groups (Figure 3).
Immunohistochemical analysis revealed that infiltration of CD163+ macrophages was greater in the upper-to-mid dermis and in the mid-to-deep dermis of ARPC (Figures 4 and 5). Both CD4+ T cells and CD8+ T cells were more abundant in the mid-to-deep dermis of ARPC (ARPC2) (Figures 4 and 5). There were few basophils in both ARPC and PN. TGF-β3 was positively stained in the upper dermis in 5 out of 22 ARPC patients (22.7%) (Figure 4), but in 1 out of 38 PN (2.6%).
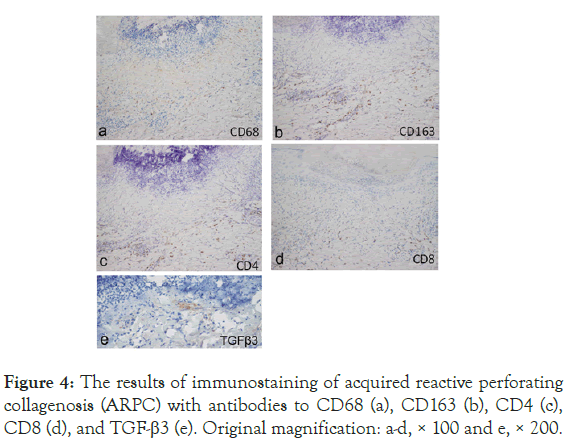
Figure 4: The results of immunostaining of acquired reactive perforating collagenosis (ARPC) with antibodies to CD68 (a), CD163 (b), CD4 (c), CD8 (d), and TGF-β3 (e). Original magnification: a-d, × 100 and e, × 200.
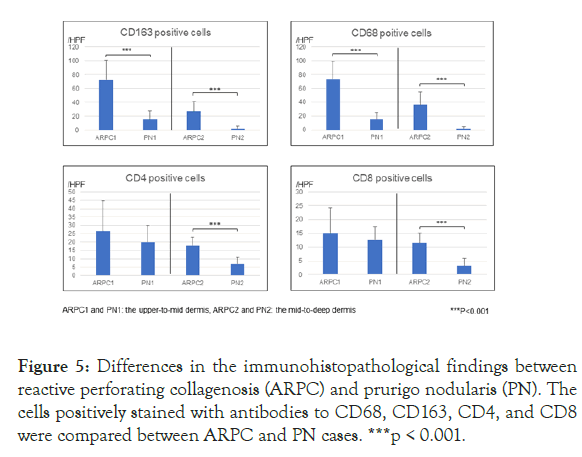
Figure 5: Differences in the immunohistopathological findings between reactive perforating collagenosis (ARPC) and prurigo nodularis (PN). The cells positively stained with antibodies to CD68, CD163, CD4, and CD8 were compared between ARPC and PN cases. ***p < 0.001.
In this study, we compared the clinical features and pathological findings of ARPC and PN. ARPC and PN show similar clinical features, i.e., itchy papules and nodules appearing in patients mostly in their 50’s and 60’s, often with systemic comorbidities. Comorbidities reported in ARPC cases include diabetes mellitus [5-9] and chronic nephropathy [7,9,10]. In the present study, diabetes mellitus was associated with both diseases, but no statistical difference between two groups. It is speculated that ARPC arises from the trauma caused by scratching, and the dermal necrosis resulting from the poor blood supply derived from diabetic vasculopathy [6]. Pathogenesis of ARPC in chronic renal failure may be associated with alterations in collagen due to metabolic disturbances and dermal microdeposits of calcium [9]. As renal failure was found in only 2 patients with ARPC in our study, the influence of renal dysfunction on the pathogenesis of ARPC may be limited.
Peripheral eosinophilia and the elevated TARC values were observed more often in PN. Eosinophil infiltration in the dermis was seen in approximately 80% of both ARPC and PN patients with peripheral eosinophilia. Histopathological analysis revealed spongiosis more often in the epidermis of PN, suggesting that PN may be caused by Th2 cytokine-induced reaction with eosinophil activation. On the other hand, the ARPC eruptions showed infiltration by neutrophils and histiocytes, and also CD163+ M2 macrophages in the dermis. Thus, our histological study suggests that the inflammation with neutrophils and M2 macrophages is involved in the pathomechanisms of ARPC. This is the first report to show the difference in the infiltrated cell profiles between the ARPC and PN.
It has been reported that the increased expression of TGF-β3 is reported in lesional skin of ARPC compared to perilesional skin [11-13]. The protein levels of matrix metalloproteases (MMPs) and tissue inhibitor of metalloproteinase-1 have been reported to be enhanced in lesional skin [11], probably by inflammatory cytokines such as TGF-β [14] and IL-1. TGF-β3 [13] and Th2 cytokines are known to activate M2 macrophages [15], which may lead to chronic inflammation and TEE in ARPC [16]. Narrow-band UV B therapy [11,17,18] and 308-nm excimer laser therapy [19] may have therapeutic effects on ARPC lesions [20] not only by inhibiting pruritus [11] and preventing neutrophil infiltration [18], but also by downregulating TGF-ß expression in fibroblasts [21]. However, in our study, expression of TGF-β3 was shown only in 22.7% of ARPC. Although less expression of TGF-β3 in ARPC lesions in this study may be caused by the effects of previous treatments such as topical steroids, it is not unclear. Thus, further study is needed to define the role of TGF-β3 in the pathomechanisms of ARPC.
Topical steroid is usually recommended for the treatment of ARPC [4,8] and the addition of emollients was found to be more effective in ARPC in our study. This may be due to improved pruritus by emollients. However, no randomized controlled studies have been conducted for ARPC [22]. As limitations of this study include the small sample size and its retrospective nature, further study is necessary to clarify the efficacy of emollients on ARPC.
This is the first report to show the histological differences in cell infiltration between ARPC and PN. The inflammation mediated by neutrophils and M2 macrophages may be involved in the formation of ARPC lesions differently from PN lesions. However, further studies are necessary to define the precise pathomechanisms of ARPC.
The authors would like to thank Mrs. Kayoko Tanaka for assisting with data collection.
The authors declare that there are no conflicts of interests.
None
Citation: Kawamura M, Inoue M, Matsuyama K, Mizutani Y, Shu E, Miyazaki T, et al. (2021) Clinicopathological Differences between Acquired Reactive Perforating Collagenosis and Prurigo Nodularis. J Clin Exp Dermatol Res. 11:550.
Received: 29-Dec-2020 Accepted: 14-Jan-2021 Published: 21-Jan-2021 , DOI: 10.35248/2155-9554.21.s8.550
Copyright: © 2021 Kawamura M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.