Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2022)Volume 13, Issue 7
Purpose: To describe the clinical and optical coherence tomography findings of Adult-Onset Foveo-Macular Vitelliform Dystrophy (AFVD) mimicking age related macular degeneration.
Method: A retrospective study design was employed which reviewed clinical and optical coherence tomography images of patients with adult-onset foveo-macular vitelliform dystrophy from September 2019 to March 2021.
Result: There were 12 (9 male and 3 female) study subjects with a mean age of 62.75 years. The presenting visual acuity ranged from 20/100 to 20/20. Of 19 eyes, 10 eyes showed vitelliform lesions; 5 eyes had pseudo-hypopyons, and 4 eyes were in the vitelliruptive stage. OCT showed disruption of the IS/OS interface in 8/19 eyes (3 eyes with stage I, 1 in stage II, and 4 eyes with stage III disease). Optically clear (non-reflective) sub retinal lesions were present in OCT images from 6 eyes (5 in pseudo-hypo yon and 1 in vitelliruptive stages). Four of five eyes in the pseudo-hypo yon stage had intact IS/OS photoreceptor interfaces.
Conclusion: An optically clear space is a distinct feature of adult-onset fovea-macular vitelliform dystrophy that is different from the pattern of fluid in endovascular AMD that may have a protective role against the disruption of the photoreceptor interface. It is important to be aware of the stages of AFVD and to differentiate them from endovascular AMD, as AFVD does not require treatment with injections of anti-vascular endothelial growth factor.
Adult-onset foveo-macular vitelliform dystrophy; Age related macular degeneration; Optical coherence tomography
Adult-Onset Foveo-Macular Vitelliform Dystrophy (AFVD) was first described by Gas in 1974 [1]. In its original description, this disease was called peculiar foveomacular dystrophy. It generally presents in the fourth to sixth decades of life in patients who are visually asymptomatic, who have mild blurring of vision, or who have mild metamorphopsia. The typical patient has bilateral, asymmetric, foveal or peritoneal, yellow, solitary, round to oval elevated sub-retinal lesions of less than 1/3 disc diameter, without surrounding drusen, often with central pigmentation [2,3]. There is a four stage classification for AFVD based on the one that was established for best vitelliform macular dystrophy. These are vitelliform, pseudo-hypoyon, vitelliruptive, and atrophic, in progressive order [4]. Many patients show a different stage in each eye, multiple characteristics of different stages within a single lesion and sometimes even multifocal vitelliform lesions [5]. The vitelliform stage is the classic dome like lesion with sub-retinal vitelliform material. In the pseudo-hypopyon stage, the vitelliform material settles inferiorly within the subretinal space, creating an optically empty zone superiorly, with hyper reflective material inferiorly and a horizontal demarcation at the interface. The vitelliruptive (scrambled egg) stage is characterized by the collapse of the dome like shape into a lesion with heterogeneous vitelliform material and hyper-reflective clumps within the inner retina. The atrophic stage is characterized by a homogenous plaque of atrophy centered on the macula. Optical Coherence Tomography (OCT) is a noninvasive technique that provides optical cross-sectional images of the retina and morphologic information similar to that obtained from histologic sections [6]. Based on spectral-domain OCT findings in patients with AFVD, it was hypothesized that early changes involve the layer between the RPE and the ellipsoid zone, first with the accumulation of material beneath the sensory retina [7,8]. Clinico-pathological studies confirmed the sub-retinal location of the vitelliform material, demonstrating pigment laden cells, lipofuscin granules, photoreceptor debris, and RPE cells in varying stages of disintegration in the subretinal space [9-11]. Due to this clinical variability of AFVD, there is considerable overlap with age related macular degeneration. Choroid Neo Vascularization (CNV) can also occur in macular dystrophies, although it is rare and appears to have a relatively favorable prognosis as compared to CNV in AMD [12]. Most often, AFVD is misdiagnosed as AMD. It is therefore important to carefully define the ophthalmoscopy and imaging characteristics of this maculopathy to improve diagnostic accuracy. The purpose of our study is to describe the clinical and OCT morphologic features in patients with AFVD that help differentiate from NV-AMD.
A retrospective study design was employed which reviewed the clinical and imaging studies of patients who have had an evaluation at WGGA eye center and Roha specialized eye clinic, in Addis Ababa, Ethiopia from September 2019 to March 2021. Inclusion criteria were all patients with a clinical diagnosis of AFVD, confirmed by the presence of a sub-foveal, round, yellowish lesion and with the corresponding presence of hyperreflective sub-retinal material on Spectral-Domain Optical Coherence Tomography (SD-OCT). Five fellow eyes with no apparent macular lesions were excluded. All study subjects had a comprehensive ophthalmology examination including measurement of Best-Corrected Visual Acuity (BCVA) at 3 M with Snelling’s visual acuity chart, intraocular pressure with Topcon non-contact tonometry, dilated bio-microscopic examination, fundus photography, and optical coherence tomography using 3D OCT-1, Maestro-I/and II, Topcon, Tokyo, Japan. The field of view of 3D OCT-1 used was a 6 mm by 6 mm area centered on the fovea. Nineteen eyes had macular scans, and both 3D and 2D cross-sectional images were assessed with particular attention to the inner segment/outer segment (IS/OS) of photoreceptor cells, the retinal pigment epithelium, the morphology, and the stage and location of vitelliform lesions.
There were 12 (9 M and 3 F) study subjects with a mean age of 62.75 years. Cases 1, 2, 3, 4, 6, 7, 9, 10, 11 and 12 noticed a gradual blurring of vision, while case 5 presented with sudden onset of distortion of vision in the right eye. Case 8 had no complaint of reduced vision, and the lesions were detected during a routine fundus evaluation shown in Table 1.
| Age/gender | Study eye | VOD | VOS | Integrity of IS/OS interface OD | Integrity of IS/OS Interface OS | Stage OD | Stage OS |
|---|---|---|---|---|---|---|---|
| 1. 67/M | OU | 20/50 | 20/40 | Diffuse disruption | Focal disruption | III | II |
| 2. 63/M | OU | 20/70 | 20/70 | Focal disruption | Focal disruption | III | III |
| 3. 82/M | OU | 20/60 | 20/100 | Disrupted | Disrupted | I | I |
| 4. 70/M | OS | NA | 20/60 | NA | Disrupted | NA | I |
| 5. 58/F | OD | 20/20 | NA | Intact | NA | II | NA |
| 6. 59/M | OD | 20/30 | NA | Intact | NA | II | NA |
| 7. 64/F | OS | NA | 20/25 | NA | Intact | NA | I |
| 8. 55/M | OS | NA | 20/70 | NA | Segmental disruption | NA | III |
| 9. 40/M | OU | 20/60 | 20/20 | Intact | Intact | II | I |
| 10. 54/M | OU | 20/20 | 20/30 | Intact | Intact | I | I |
| 11. 70/M | OU | 20/60 | 20/60 | Intact | Intact | I | I |
| 12. 71/F | OU | 20/100 | 20/30 | Intact | Intact | I | II |
Note: NA is to indicate eyes that were excluded as they did not have AFVD lesions.
Table 1: Patient characteristics.
The presenting visual acuity ranged from 20/100 to 20/20. Seven study subjects had bilateral disease, while five were unilateral. Their five fellow eyes were excluded from analysis because they did not have yellow macular deposits or other signs of AFVD. Nineteen eyes fulfilled clinical and OCT inclusion criteria for AFVD. There were 10 eyes with vitelliform lesions (stage I); 5 pseudo-hypopyon eyes (stage II); 4 vitelliruptive eyes (stage III) and no atrophic stage eyes (stage IV). OCT through the macular lesion demonstrated optically clear spaces in all 5 eyes with pseudo-hypopyon stage and in 1 eye with vitelliruptive stage (case 2 right eye). OCT showed disruption of IS/OS of photoreceptors in 8/19 (42%) of study eyes (3 eyes with stage I, 1 eye with stage II, and 4 eyes with stage III disease). It was evident that both the inner segment/outer segment (IS/OS) interface and the External Limiting Membrane (ELM) were disrupted in two eyes (Case 1, OS and case 2, OD), and as a result of this, hyper-reflective clumps were seen in the inner retina shown in Figures 1 and 2.
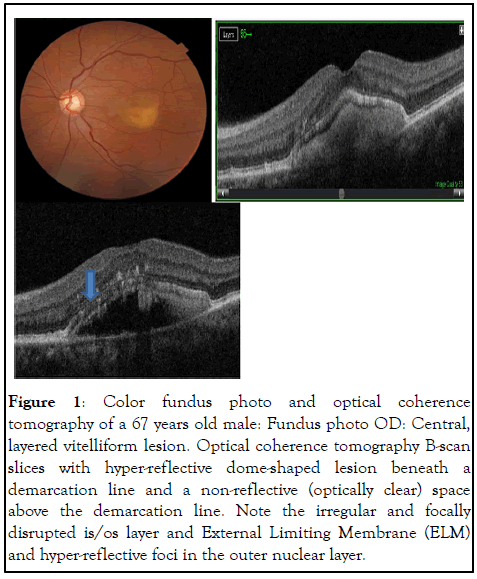
Figure 1: Color fundus photo and optical coherence tomography of a 67 years old male: Fundus photo OD: Central, layered vitelliform lesion. Optical coherence tomography B-scan slices with hyper-reflective dome-shaped lesion beneath a demarcation line and a non-reflective (optically clear) space above the demarcation line. Note the irregular and focally disrupted is/os layer and External Limiting Membrane (ELM) and hyper-reflective foci in the outer nuclear layer.
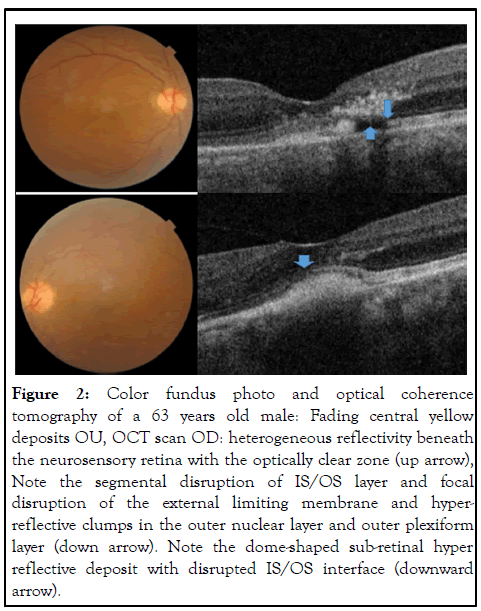
Figure 2: Color fundus photo and optical coherence tomography of a 63 years old male: Fading central yellow deposits OU, OCT scan OD: heterogeneous reflectivity beneath the neurosensory retina with the optically clear zone (up arrow), Note the segmental disruption of IS/OS layer and focal disruption of the external limiting membrane and hyperreflective clumps in the outer nuclear layer and outer plexiform layer (down arrow). Note the dome-shaped sub-retinal hyper reflective deposit with disrupted IS/OS interface (downward arrow).
In three eyes (cases 3 and 4), fundus photography showed multiple round yellowish macular deposits, and their corresponding SD-OCT revealed discrete hyper-reflective subretinal deposits with distortion of the contour of the IS/OS layer that appears to be reticular-pseudodrusen-like lesions shown in Figures 3 and 4.
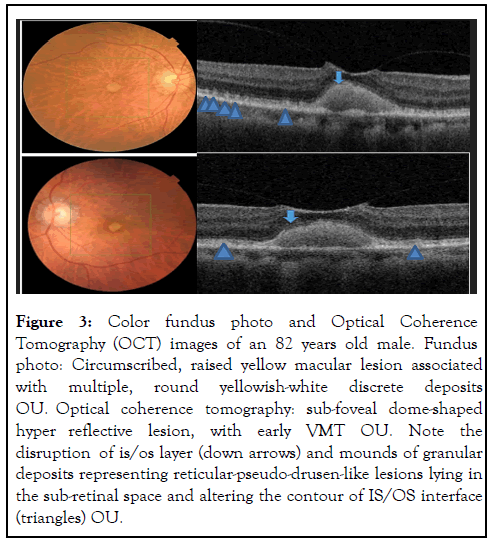
Figure 3: Color fundus photo and Optical Coherence Tomography (OCT) images of an 82 years old male. Fundus photo: Circumscribed, raised yellow macular lesion associated with multiple, round yellowish-white discrete deposits OU. Optical coherence tomography: sub-foveal dome-shaped hyper reflective lesion, with early VMT OU. Note the disruption of is/os layer (down arrows) and mounds of granular deposits representing reticular-pseudo-drusen-like lesions lying in the sub-retinal space and altering the contour of IS/OS interface (triangles) OU.
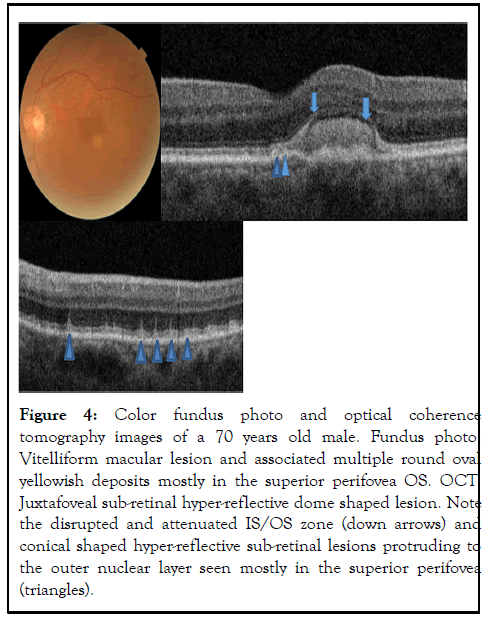
Figure 4: Color fundus photo and optical coherence tomography images of a 70 years old male. Fundus photo: Vitelliform macular lesion and associated multiple round oval yellowish deposits mostly in the superior perifovea OS. OCT: Juxtafoveal sub-retinal hyper-reflective dome shaped lesion. Note the disrupted and attenuated IS/OS zone (down arrows) and conical shaped hyper-reflective sub-retinal lesions protruding to the outer nuclear layer seen mostly in the superior perifovea (triangles).
The term adult-onset foveomacular vitelliform dystrophy currently used in the literature likely represents a group of disorders with overlapping phenotypes [13]. Some cases have an autosomal dominant trait while similar phenotypes are explained by a variety of genetic, toxic, immune-mediated, degenerative, and mechanical mechanisms. Optically clear (nonreflective) subretinal lesions were present in OCT images from 6/19 eyes (5 in pseudo-hypopyon and 1 in vitelliruptive stages). It is worth calling attention to the OCT image of case 1, OS shown in Figures 1 and 2, with a hyper-reflective dome beneath the demarcation line and an overlying optically clear space. This is a pseudo-hypopyon stage representing partial liquefaction of vitelliform material. This optically clear space is a distinct feature of AFVD different from the pattern of fluid seen in neo-vascular AMD. The sub-retinal fluid in neo-vascular-AMD assumes a more horizontal position as there is a generalized macular disease and less resistance for the fluid to spread in the interstitial space of the retina. It is also associated with neovascular membranes seen by SD-OCT as a hyper-reflective band/or patch beneath the retinal pigment epithelium, in the subretinal space, or bridging the RPE and retina. Intra-retinal fluid can be seen based on the stage of the disease and the type of choroidal endovascular membrane. In AFVD, optically clear cystic spaces lying between the retinal pigment epithelium and the neurosensory retina conform to the dome-shaped lesion. This optically clear region was also considered to represent a loss of the photoreceptors and/or accumulation of eosinophilia fluid, as was reported by Jaffe and Schatz. In addition, the accumulation of sub-retinal fluid may be due to the decreased ability of RPE cells to pump fluid from the sub-retinal space. In vitelliform macular dystrophy, the sub-retinal fluid appears to be caused by an underlying defect of VMD2, which encodes bestrophin, a calcium-sensitive chloride channel protein found in the basolateral membrane of RPE cells [14]. In contrast to AMD in which histopathological abnormalities commence as basal laminar deposits beneath the RPE, the earliest changes of AFVD involve the photoreceptors. The SD-OCT findings in our series also confirm the location of the vitelliform material in the sub-retinal space lying between the photoreceptors and retinal pigment epithelium. Another interesting observation was that 4/5 of eyes with the pseudo-hypopyon stage had intact IS/OS interfaces. Cone photoreceptors can function relatively well despite the physical separation from the RPE because of mechanisms such as an alternative, intraregional visual pigment regeneration route. The classic pigment regeneration route involves photo isomerization of 11-cis-retinal to all-trans-retinal in the photoreceptors and the shuttling of all-trans-retinol from the photoreceptors to the RPE and 11-cis-retinal from the RPE to the photoreceptors. Recent evidence suggests the existence of a second retinoid visual cycle within the retina that is independent of RPE to specifically support rapid cone pigment regeneration [15,16]. The sedimentation of vitelliform material inferiorly, and the resulting optically clear space between the material and the IS/OS interface of photoreceptors may shield the IS/OS interface from the effect of the vitelliform material. Therefore, we hypothesize that the presence of fluid in the subretinal space could have a protective role against the disruption of the IS/OS of photoreceptors at least until the disease progresses to the next stage. We understand this has to be proven through a long-term, prospective study with enough study subjects. It is important to note the SD-OCT findings of case 1 (OS) and case 2 (OD) with pseudo-hypopyon and vitelliruptive stages where hyper reflective clumps were seen in the outer nuclear and outer plexiform layers. These lesions in the inner retinal layer may correspond to migrated pigment laden cells that gained access through the disrupted IS/OS and external limiting membrane layers, which is in agreement with previous OCT and clinicopathologic reports. Such hyperreflective dots may reflect part of an absorption process of the vitelliform lesion and may not represent lesions of AMD. The sudden onset of metamorphopsia noticed in the right eye of case 5 could be the result of an abrupt collapse of the vitelliform lesion that produced morphologic changes in the neurosensory retinal layer. Careful monitoring of this patient showed no sign of choroidal neovascularization, and there was complete resolution of complaints in subsequent weeks. In our series, SDOCT images showed disruption of the IS/OS interface in 8/19 (42%) of study eyes. Interestingly, 3/19 eyes with disrupted IS/OS layers had vitelliform lesions, the first stage of the natural course of the disease, and associated reticular-pseudo-drusen like lesions. Although most eyes in the vitelliform stage have preserved vision, some eyes could develop disruption of the IS/OS interface in the early stage of AFVD. One or any combination of the descriptions could explain how some eyes in the early stage of AFVD develop the disruption of the ellipsoid zone.
• Although we could not make any assumptions on how long the vitelliform material had been in the sub-retinal space, we think that even in the early stage of AFVD, there could be local phagocytic dysfunction of RPE. And as a result of this functional failure, pigment-laden cells and other components of vitelliform material could cause damage to the photoreceptors.
• The presence of reticular-pseudo-drusen like deposits could have contributed to mechanisms that led to the disruption of IS/OS of photoreceptors described in 3 eyes.
• Moreover, previous studies explained the challenge of delineating the IS/OS interface, due to the elevated domeshaped lesions. Alternatively, it has been reported in a clinic pathological study of AFVD eyes that inner segments may be stunted, which may represent a sign of photoreceptor cell dysfunction.
AFVD was associated with different types of drusen that include subretinal drusenoid deposits (reticular pseudodrusen), basal laminar deposits (cuticular drusen), or drusen that are typical for AMD. Reticular psuedodrusen has been reported in diseases whose pathophysiological mechanisms primarily involve damage to the Bruch’s membrane and the RPE. The prevalence of these sub-retinal deposits in newly presenting AFVD was 40% [17]. The subretinal location of both psuedodrusen and Vitelliform lesions might reflect a common pathogenic mechanism associated with the altered function of the RPE-photoreceptor complex. As we had a small sample size, we were not able to correlate the visual acuity reduction to disruption of the IS/OS of photoreceptors. Overall, the level of visual acuity reduction was not profound, in contrast to the visual acuity reduction in age related macular degeneration which tends to be more significant. This observation is compatible with other reports.
In our case series, we described the distinct pattern of the fluid in AFVD. Based on the finding of intact IS/OS interface in eyes with most pseudo-hypopyon stages, we hypothesize that the presence of fluid that shields the photoreceptor cells from the effect of vitelliform material could have a protective role from disruption of IS/OS interface. It is important to be aware of pseudo-hypopyon and vitelliruptive stages of AFVD that have sub-retinal fluid and hyper-reflective intra-retinal changes and to differentiate this condition from neo-vascular AMD. Making this distinction can help avoid unnecessary interventions and risks associated with intravitreal treatment.
[Crossref][Googlescholar][Indexed].
Citation: Tesfaw AK, Bernstein PS (2022) Clinical and Optical Coherence Tomography Features of Adult-Onset Foveo-Macular Vitelliform Dystrophy Mimicking Age-Related Macular Degeneration. J Cli Exp Ophthalmol. 13:935.
Received: 17-Jun-2022, Manuscript No. JCEO-22-17981; Editor assigned: 18-Jun-2022, Pre QC No. JCEO-22-17981(PQ); Reviewed: 02-Jul-2022, QC No. JCEO-22-17981; Revised: 18-Aug-2022, Manuscript No. JCEO-22-17981(R); Published: 02-Sep-2022 , DOI: 10.35248/2155-9570.22.13.935
Copyright: © 2022 Tesfaw AK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.