Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2020)
Background and aim: Chronic Hepatitis C (CHC) remains the most common cause of Hepatocellular Carcinoma (HCC). Direct-Acting Antiviral (DAA) therapy reduce incident of HCC among HCV-infected patients, however the impact of these therapies on tumor behavior is less clear. Here, we compared the characteristics of HCC diagnosed before and after initiation of f DAA therapy in large population-based cohort.
Patients and methods: In a prospective cohort of an outreached program in 73 villages across Egypt, 14,495 (91.2%) patients were treated with DAAs and followed after SVR for median of two years (12-45 months), Of those, 275 patients had HCC (166 patients before and 109 patients after the initiation of DAA therapy).
Results: Patients who developed HCC after DAA had less tumor size, portal vein invasion, advanced stage according to BCLC classification and Milan criteria compared to those who developed HCC before therapy (P<0.05, for all comparisons). These findings remained significant independent of age, sex, body mass index (BMI), AFP, viral load and Child-Pugh score (Odds ratio: 0.338; 95% confidence interval: 0.13-0.366; P=0.0001).
Conclusion: HCC developed in CHC patients who achieved SVR following DAAs tend to display less aggressive pattern when compared to HCC diagnosed before DAAs therapy.
Chronic hepatitis C; Hepatocellular carcinoma; Antiviral Therapy; Direct-acting antivirals
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers globally, with a poor prognosis and a leading cause of cancerrelated death that occurs predominantly in patients with underlying significant liver fibrosis and cirrhosis. Chronic hepatitis C virus (HCV) infection is still a major public health concern and it is the most common cause of hepatocellular carcinoma (HCC), with an annual incidence of HCC is approximately 3-8% in patients with cirrhosis [1].
The availability of highly effective direct-acting antivirals (DAAs) with excellent safety profiles, even in patients with advanced hepatic fibrosis and liver cirrhosis has completely revolutionized the treatment landscape of patients with chronic hepatitis C (CHC) and led to that the treatment of patients with CHC being dramatically scaled up in last years, with the potential to attenuate HCV-related HCC incidence [1]. However, a high proportion of CHC patients remain undiagnosed, with estimation that nearly half of patients with HCV are currently unaware of their infection. Furthermore, the suboptimal rates of HCV treatment render this unlikely in the near future. Hence, the HCV-related HCC incidence could continue to grow at least over the next decade, if not longer [2].
Notably, an association between DAA and HCC is emerging, although details of this interaction are still unclear, but clarifying it is of pivotal clinical utility [3]. The dynamic impact of DAAs on the risk of HCC in patients with CHC, the characteristics and aggressiveness of HCC and how the risk of HCC varies over time in patients who receive DAAs compared to those who do not remains unclear. On the other hand, nearly one fourth of patients with active HCC have unrecognized HCV at time of tumor diagnosis and those subjects can be managed with DAA. In particular, the characteristics of HCC developed after DAA therapy compared to those developed before DAA therapy are still unknown. A couple of retrospective reports based on small size series suggested an alarming data that HCC may present with an aggressive pattern after DAA therapy [4,5]. These worrying findings necessitate clarification in large prospective cohorts of patients.
The Educate, Test and Treat programmer is a large prospective cohort of patients were screened and treated with DAA for HCV, with a systematic data collection, thus allowing an accurate assessment of predefined outcomes that cover the whole spectrum of interaction between DAA and HCC in this population [6]. Utilizing this cohort, we sought to examine for difference in clinical and tumor characteristics of patients with HCC diagnosed before and following DAA treatment and account for the influence of potential confounders.
Patient cohort
This is a prospective cohort study that included patients with HCV recruited from those enrolled in the Educate, Test and Treat program, which was implemented in 73 Egyptian villages, the details of this cohort have been recently described [6]. Briefly, the Educate, Test and Treat program was conducted in the outpatient clinics of the Egyptian Liver Research Institute and Hospital (ELRIAH) and its satellites from January 2015 until August 2017. In total, 310,814 patients were targeted. Of those, 221,855(71.4%) were found to be eligible for screening, and 204,749(92.3%) were screened for antibodies to HCV, followed, in patients who tested positive, by PCR for HCV RNA. In total, 15,892 individuals were found to be positive for HCV RNA and underwent full assessments. 3192(20.1%) had cirrhosis.
Patients with either hepatitis B virus (HBV) or human immunodeficiency virus (HIV) co-infection or with a history of previous interferon (IFN) treatment, decompensated cirrhosis, liver transplantation, renal impairment, and other malignancies or nonmalignant hepatic focal lesions (dysplastic nodules, cirrhotic nodules, and haemangiomas) were excluded.
Patient evaluations and follow-up
All patients were evaluated before initiation of antiviral treatment. After the start of antiviral treatment, patients were seen by physicians every 4 weeks until the end of therapy and then again 12 weeks after the end of therapy to assess SVR (SVR12). Patients were followed up every 6 months for at least 1 year from the end of treatment. When patients did not attend a planned follow-up assessment, the village promoters were informed, and they contacted the patients.
The screening assessment included the usual clinical and biological parameters, including sex; age, BMI and routine laboratory tests. BMI was calculated as weight divided by the square of the height (kgm2). Routine haematological and biochemical laboratory tests were performed at each follow-up visit and determined by ELRIAH laboratories. They included the full blood count, liver transaminases (alanine aminotransferase (ALT) and aspartate aminotransferase (AST), AFP, serum creatinine, serum albumin, serum bilirubin, HCV HCV-RNA testing was performed with use of a real-time HCV RNA PCR (Cobas Ampliprep, Cobas Taqman 48, Roche, Basel, Switzerland) according to the manufacturer’s instructions.
Diagnosis and staging of liver fibrosis
Abdominal ultrasonography was performed at each follow-up visit using a Toshiba Aplio XG ultrasound machine (Toshiba, Minato, and Tokyo, Japan). Transient elastography was performed with a FibroScan 502 (Echosens, Paris, France) and three portable Echosens Mini Systems (Echosens, Paris, France) before treatment, 1 year after the end of treatment, and at the yearly follow-up visits to determine the stage of fibrosis. Patients were diagnosed as having liver cirrhosis (F4) when they fulfilled more than one of the following criteria: definite clinical signs and laboratory parameters of liver cirrhosis; abdominal ultrasonographic signs suggestive of cirrhosis; transient elastography suggestive of cirrhosis (>16.3 kPa) [7]; or non-invasive scores suggestive of cirrhosis (FIB-4>3.25 and APRI>1.0).
Patients were diagnosed as having advanced liver fibrosis (F3) mainly by: transient elastography (>10.2 and ≤ 16.3 kPa) and non-invasive scores (FIB-4 >1.45 and ≤ 3.25 [7,8] or APRI >0.7 and ≤ 1.0 [9]. Patients with cirrhosis (F4) were diagnosed according to the Child-Pugh classification [10].
Diagnosis of HCC
Diagnosis of HCC was made according to the American Association for the Study of Liver Diseases (AASLD) recommendations [11]. Triphasic MSCT or MRI was performed for patients who had AFP levels higher than 20 ng/mL or focal hepatic lesions on ultrasound. Confirmation of HCC was based on the characteristic arterial enhancement and early washout in the delayed phase of MSCT [12]. Color Doppler imaging was used to diagnose venous thrombosis in the portal vein. HCC was staged according to the Barcelona Clinic Liver Cancer (BCLC) classification system [13,14]. HCC was also classified according to the Milan criteria [15]. The threshold Milan criteria are as follows: one lesion smaller than 5 cm or up to three lesions, each smaller than 3 cm; no extrahepatic manifestations; and no evidence of gross vascular invasion.
Antiviral treatment
All participants received a 12 or 24 weeks course of one of several DAA regimens after baseline assessment, in accordance with Egyptian national treatment guidelines, the AASLD 2014, and the 2014 WHO guidelines [16,17]. Patients who experienced treatment failure received another course of DAAs (different regiment) until they achieved SVR12. Treatment included sofosbuvir and ribavirin (51.0% of patients), sofosbuvir and daclatasvir (27.3%), sofosbuvir, daclatasvir, and ribavirin (14.1%), ombitasvir, paritaprevir, and ritonavir with or without ribavirin (5.5%), and sofosbuvir and ledipasvir with or without ribavirin (2.1%) for 12 or 24 weeks [6].
Ethics
The study protocol was approved by the Research and Ethics Committee of ELRIAH. The protocol and conduct of the study complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects with its amendments in 2008. All patients gave written informed consent before initiation of the study [18]. The study was registered at https://clinicaltrials.gov under the number NCT03884062.
Statistical analysis
Statistical analyses were performed using SPSS version 24 (IBM, USA). Continuous variables are reported as the medians (interquartile range (IQR)). Categorical variables are reported as the frequencies (%). Non-parametric tests, namely, the Mann- Whitney U test for quantitative comparisons and Fisher’s exact test for qualitative comparisons, were used. Multivariable regression modeling with backward elimination was undertaken to test independent associations of the time of HCC development (before or after DAA therapy) with the following outcome variables: (a) tumor size; (b) portal vein invasion; (c) BCLC staging and (d) Milan criteria using logistic regression models to examine binary traits; tumor size dichotomized as small (≤ 3) versus large >3); portal vein invasion was dichotomized as absence or presence, BCLC staging (0/A) vs (B/D) and Milan criteria (<5 cm and ≥ 5 cm). Analyses were adjusted for biologically relevant covariates and potential confounders associated with the risk of HCC aggressiveness (age, sex, BMI, viral load, AFP and Child-Pugh score).
275 patients were enrolled in the study: 166 developed HCC before the start of DAA treatment and 109 CHC patients who developed HCC after achieving SVR following to DAA treatment during a follow up period of 23.51 ± 8.21 months (range 12-45 months).
Out of those who developed HCC after therapy, 11 (10.1%) occurred during the first year of follow-up, 53(48.6%) during the second year, 35(32.1%) during the third year, and 10(9.2%) occurred after the third year of follow-up (Figure 1). Accordingly, cumulative incidence of HCC was 0.4%, 2.7%, 4.2% and 4.6% at 1, 2, 3 and 3.8 years after the end of DAA therapy.
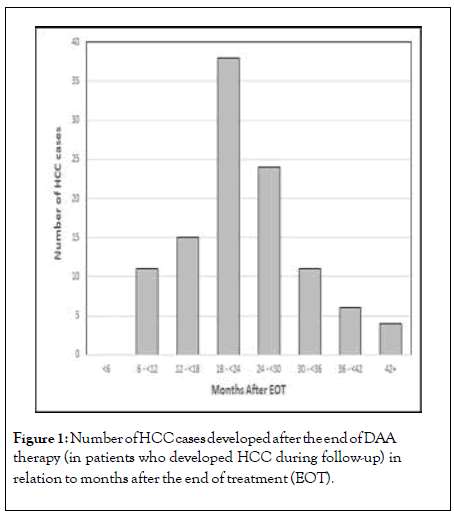
Figure 1: Number of HCC cases developed after the end of DAA therapy (in patients who developed HCC during follow-up) in relation to months after the end of treatment (EOT).
Patient and laboratory characteristics
Patients who developed HCC before DAA treatment were significantly older [62(IQR 57.5-67.5) years vs 59 years (IQR 55.5- 65.0); p=0.028] with a male predominance (92.8% vs 75.2%; p<0.001) and more obese (defined as BMI ≥ 30 kg/m2) (55% vs 36.7%; p=0.003) compared to those who developed HCC after DAA treatment (Table 1).
| Variable | HCC diagnosed before DAAs (n=166) | HCC diagnosed following DAAs (n=109) | p value |
|---|---|---|---|
| Sex: Male(%) |
154(92.8%) | 82(75.2%) | <0.001 |
| Age(years), Median(IQR) | 62.0(57.5-67.5) | 59.0(55.5-65.0) | 0.028 |
| AFP group at diagnosis -<20 ng/mL -29 ng mL to <100 ng/mL -100 ng/mL to <1000 ng/mL -≥1000 ng/mL Median(IQR) |
51(30.7%) 3(1.8%) 27(16.3%) 85(51.2%) 726(7.8-3440.5) |
42(38.5%) 24(22.0%) 27(24.8%) 16(14.7%) 31.0(12.1-731.3) |
<0.001 <0.001 |
| Child-Pugh stage at HCC diagnosis -A -B -C |
132(79.5%) 29(17.5%) 5(3%) |
84(77.1%) 15(13.8%) 10(9.2%) |
0.074 |
| Fibrosis stage before HCV treatment -F3 -F4 -FIB4, Median(IQR) -APRI, Median(IQR) |
0(0.0%) 166(100.0%) 6.14(4.66-8.25) 1.79(1.27-2.90) |
8(7.3%) 101(92.7%) 5.69(4.18-7.82) 1.51(0.89-2.77) |
<0.001 0.002 0.006 |
| HCV viremia before treatment (Log 10) | 5.36(4.7-6.0) | 5.38(4.57-5.95) | 0.329 |
| Comorbidities -Diabetes mellitus -Hypertension -Overweight# -BMI -ALT -AST -ALP |
36(21.7%) 15(9.0%) 61(36.7%) 27.2(23.1-31.2) 67.0(64.5-78.5) 78.0(54.0-78.5) 273.0(203.0-444.0) |
25(22.9%) 17(15.6%) 60(55.0%) 30.8(26.6-34.6) 48.5(36.5-80.5) 60.0(40.0-100.0) 124.5(67.5-515.0) |
0.807 0.097 0.003 0.015 0.002 0.007 <0.001 |
Data are presented as the frequency (%) or median (IQR). HCC:Hepatocellular carcinoma; DAAs: Direct-acting antivirals; IQR: Interquartile range; AFP:Alpha-fetoprotein; HCV:Hepatitis C virus.
#Overweight (BMI ≥ 30 kg/m2).
Table 1: Demographic and laboratory characteristics of patients with HCC.
AFP levels were significantly higher in patients who developed HCC before DAA treatment (51.2% were higher than 1000 ng/mL) than in those who developed HCC after DAA treatment (14.7% higher than 1000 ng/mL; p<0.001). While, no significant differences were noted between the two groups regarding HCV viremia, diabetes mellitus, hypertension, and Child-Pugh stage.
Tumor characteristics
Although, the number of tumors did not differ significantly (p=0.2), the tumor size was significantly less in those who developed HCC after therapy compared to those who developed before (p=0.040). This finding remained significant in multiple logistic regression analysis adjusting for of age, sex, Bod mass index (BMI), AFP, viral load and Child-Pugh score (Odds ratio: 0.174, 95% confidence interval (95% CI): 0.015-1.02, p=0.04). In addition, the tumor occurred in both lobes in 42.2% of patients with HCC before DAA treatment and in only 32.1% of patients who developed HCC after DAA treatment (p<0.001). Similarly, the presence of portal vein invasion was more frequent in patients who developed HCC before compared to those who developed after HCC (44% vs 7.3, p=0.001). This finding remained significant in multiple logistic regression analysis adjusted for the same variables listed above (OR: 0.338; 95% CI: 0.13-0.366; P=0.0001) (Table 2).
| Variable | HCC diagnosed before DAAs | HCC diagnosed following DAAs | p value |
|---|---|---|---|
| Tumour number -Single -Multiple |
60(39.2%) 101(60.8%) |
51(46.8%) 58(53.2%) |
0.210 |
| Tumour size cm - ≤ 3 - ≥ 3 |
60(36.1%) 106(63.9%) |
53(48.6%) 56(51.4%) |
0.040 |
| Tumour laterality -Right -Left -Both lobes |
92(55.4%) 4(2.4%) 70(42.2%) |
51(46.7%) 23(21.1%) 35(32.1%) |
<0.001 |
| Portal vein invasion -No -Yes |
92(55.4%) 74(44.6%) |
101(92.7%) 8(7.3%) |
<0.001 |
| BCLC criteria -0 -A -B -C -D |
36(21.7%) 29(17.5%) 27(16.3%) 60(36.1%) 14(8.4%) |
8(7.3%) 35(32.1%) 33(30.3%) 29(26.6%) 4(3.7%) |
<0.001 |
| Milan criteria -Beyond criteria -Within criteria ---Single ≤ 5 cm --- ≤ 3 lesions ≤ 3 cm |
142(85.5%) 24(14.5%) 13(54.2%) 11(45.8%) |
70(64.2%) 39(35.8%) 22(56.4%) 17(43.6%) |
<0.001 |
Table 2: Characteristics of HCC tumors in the studied patients.
BCLC classification
Then we assessed if the time of development of HCC would also correlate with tumor aggressiveness according to BCLC classification. We demonstrated that 21.7%, 17.5%,16.3% and 8.4% of patients who developed HCC before DAA therapy had stage 0, stage A, stage B, and stage C, respectively; while 7.3%, 32.1%,, 30.3%,, 26.6% and 3.7% of patients who developed HCC after DAA therapy had stage 0, stage A, stage B, and stage C, respectively. The difference between the two groups was significant (p<0.001), though has not maintained in multiple logistic regression analysis.
Milan criteria
Milan criteria are currently the most popular method for consideration of patients with HCC for liver transplantation. Thus, we finally, explored the association between DAA therapy and Milan criteria. Consistently, patients who developed HCC before DAA therapy have more advanced Milan criteria score compared to those who developed after initiation of DAA (P=0.001) (Table 2). This finding remained significant in multiple logistic regression analysis adjusted for the same variables listed above (OR: 0.203; 95% CI: 0.032-0.304; P=0.01).
To the best of our knowledge, this is the first study to examine the clinical presentation and tumour characteristics in patients that developed HCC before and after DAA treatment in a large-scale prospective cohort. The key finding of this work is that clinical and tumour characteristics of HCC developed after treatment display less aggressive phenotype compared to that developed HCC before therapy.
To elaborate, we demonstrated that HCC that developed after treatment were significantly less likely to be presented with large size, multi-lobular tumor or to show the presence of vascular invasion of the portal vein or its branches. Similarly, they were less likely to present with intermediate/ advanced stages, as assessed by BCLC sore or Milan criteria. Notably, these findings remained significant after adjusting for potential biological confounders such as age, sex, BMI, viral load and Child-Pugh stage.
Even more importantly, approximately 7.5% of the overall post DAA-related HCC cases arise in the absence of cirrhosis in our cohort, while all of patients who developed HCC before therapy were cirrhotic (P<0.001). Therefore, it is plausible that carcinogenesis can occur in post-DAA in the absence of cirrhosis, although, as expected, the incidence (0.64 per 100 patient-years) was much lower than in patients with pre- treatment cirrhosis. The precise estimate of the residual risk of HCC after SVR will be pivotal to determine the utility of HCC surveillance and to recommend HCC screening after SVR for DAA. Furthermore, with scaling up of HCV treatment and the fact that most patients have been already treated or will undergo HCV treatment with DAAs in the near future, and virtually all will develop SVR, further studies to identify biomarkers that can reliably recognize at-risk subgroups of patients among the general population will be required.
The oncogenic mechanisms involved in the progression of HCC after DAA remain to be identified, but our data suggest that is potentially different oncogenic pathways drive the progression of HCC after DAA treatment and they thus result in distinct tumor phenotypes. The rapid clearance of the virus with subsequently impaired immune surveillance leading to occurrence of HCC was one of the early postulated mechanisms [19]. However, our findings as well as other several large prospective studies that showed that the incidence and recurrence of HCC are decreased following DAA therapy and the fact that most of the HCC cases occurred during the second and or third year after treatment with DAAs are not supporting this hypothesis [20-23]. Furthermore, the HCV-viremia levels were demonstrated to be not significantly different between subjects who developed HCC before and after DAA treatment. This further suggests a distinct pathway of carcinogenesis after DAA therapy that is not dependent on HCV-viremia and related immune mechanisms. Recently, epigenetic changes have been suggested as potential mechanism [24]. Though, the precise mechanisms at work are under active scrutiny.
The current study has several strength and limitations. The main strength of this study is including a large sample size, prospective cohort of real-life data (n=3192), with a follow-up up to 4 years. Moreover, our work added information not only about the characteristics and vascular invasion of HCC that develops following DAA therapy but also regarding the timing of occurrence in relation to therapy, providing more accurate data about the aggressiveness of the tumor.
Our current study has also some limitations. It is hard to ascertain the time of HCC development and thus might be a lead time bias impacting the current results. However, our findings remained significant after adjusting for other potential confounding variables. Although, ideal was to compare a cohort of patients with equal time of HCC development, however, this would be unethical not to treat patients with safe, highly effective readily available DAAs. At the minimum, our work can serve as a proof of concept and will require further confirmation. In addition, this study is a single-centre study, thus further studies in other population are required to validate the current findings.
In conclusion, HCC that developed in CHC patients who achieved SVR following DAA therapy did not display an aggressive pattern compared to HCC diagnosed before DAA therapy. These tumors did not usually occur in the early months after end of treatment.
Citation: Shiha G, Amer T, Mikhail NNH, Soliman R, Elbasiony M, Gad D, et al. (2020) Characterization of Hepatocellular Carcinoma Following Direct- Acting Antiviral Therapy: A Prospective Study. J Antivir Antiretrovir.12:202. DOI: 10.35248/1948-5964.20.12.202
Received: 15-Jun-2020 Accepted: 30-Jun-2020 Published: 07-Jul-2020
Copyright: �?�© 2020 Shiha G, et a.l. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.