Journal of Osteoporosis and Physical Activity
Open Access
ISSN: 2329-9509
ISSN: 2329-9509
Review Article - (2017) Volume 5, Issue 2
In the last few years, research on the use of biochemical markers of bone turnover has greatly improved. Among the several available bone turnover markers, monitoring serum C-terminal cross-linked telopeptides is one of the most accurate one. Manual and automated immunoassays are available for the measurement of CTX, which show high analytical performance. However, they are very expensive, time-consuming and require technical experts to perform the CTX assay. Different research groups have proposed novel immunosensing methods to detect CTX biomarker. It is a hope that the development of a rapid and inexpensive point-of-care device can aid in monitoring the bone metabolism more frequently, which can be helpful in indicating the early stages of bone loss.
<Keywords: CTX; Osteoporosis; Bone marker; ELISA; Biosensor.
Osteoporosis is a common disease that is usually silent until the first fracture occurs and has a noticeable effect on the individuals and the health economics in the developed countries [1-3]. According to Osteoporosis Australia’s report that was released in 2013, 1.2 million Australians were estimated to have osteoporosis and 6.3 million individuals were diagnosed with low bone density.
In 2013, there was one fracture every 3.6 min in Australia and it was predicted there would be one fracture every 2.9 min by 2022. Moreover, in this report the total healthcare cost associated with diagnosis and management of osteoporosis was estimated to be $33.6 billion, over the next ten years [4].
According to the World Health Organization (WHO), Dual X-ray Absorptiometry (DXA) is currently the gold standard and the most accurate technique for the diagnosis of osteoporosis [5]. However, bone mineral density (BMD) studies are required to be done in longer intervals between the measurements due to the slow changes in BMD. Compared to the standard tools of BMD measurement that requires at least two years to show a proper response with starting or stopping of therapy, biochemical markers respond more quickly, within approximately a few months [6,7].
Biochemical markers of bone turnover
Biochemical monitoring of bone loss depends on the measurement of proteins and enzymes released during the process of bone resorption and bone formation. Several biochemical markers are available, which allow a sensitive and specific monitoring of bone metabolism.
Although the biochemical markers are not suggested to use in osteoporosis diagnosis yet, but they are useful for the early detection of bone loss and monitoring the response to therapy [8].
Biochemical markers of bone turnover can give more real-time information about the bone formation, resorption and turnover [6-9]. Markers of bone metabolism can be divided into two broad categories: bone resorption markers and bone formation markers, which indicate the activity of the osteoclast and the osteoblast, respectively [10-12].
| Bone formation | Bone resorption |
|---|---|
| By products of collagen synthesis Procollagen type I C-terminal (s) propeptide (s) Procollagen type I N-terminal (s) propeptide (s) |
Collagen degradation products Hydroxyproline (u) Pyridinoline (u,s) Deoxypyridinoline (u,s) |
| Matrix protein Osteocalcin (s) |
Cross-linked telopeptides of type I collagen N-terminal cross-linked telopeptide (u,s) C-terminal cross-linked telopeptide (u,s) |
| Osteoblast enzyme Total alkaline phosphatase (s) Bone alkaline phosphatase (s) |
Osteoclast enzymes Tartrate-resistant acid phosphatase (s) Cathepsin K (s) |
Table 1: Biochemical markers of bone turnover ((s) Measured in serum; (u) measured in urine).
Most commonly used biochemical markers of bone turnover are given in Table 1 [1]. Among the various biochemical markers of bone turnover, measurements of urinary and serum C-terminal telopeptides of Type I collagen (CTX) are the most accurate and sensitive [13,14].
CTX assay development
The serum CTX assay measures carboxyl-terminal collagen crosslinks in serum. This measurement is reported to be more sensitive and specific than other measurements [15].
Urine CTX assays
The first CTX assay was developed for the measurement of CTX in urine samples and it was a competitive enzyme-linked immunoassay [16]. Later in 1996, a coated-tube radioimmunoassay (RIA) was introduced for urine monoclonal antibody [17]. The tube surface was coated with the eight amino acid peptide (EKAHDGGR) and the same peptide was considered as the calibrator. In 1997, an ELISA was developed using the same monoclonal antibody [18].
In the developed assay the competitor antigen was coated onto the wells of a microtiter plate and a child’s urine was considered as the calibrator where the concentration was determined by the original assay [16].
Serum CTX assays
Serum CTX assays are more attractive than urinary markers of bone resorption because they do not require a second assay for the measurement of creatinine. Moreover, in serum-based assays, the specimen is more reproducible [18,19].
The first blood-based CTX assay was an ELISA with a capacitive polyclonal antibody [20]. After that, the same group developed a serum based ELISA based on two monoclonal antibodies [21,22]. The next significant step in the progress of the serum-based CTX assay was automation.
Several automated versions of serum CTX assays are reported [23-25]. The latest version of the automated assay [26] was developed based on chemiluminescence technology and demonstrated good analytical characteristics.
Development of biosensors for CTX measurement
Despite the excellent sensitivity and selectivity of the developed assays, which are mentioned in the last section, they have some limitations. These methods are very expensive and time-consuming. Furthermore, they are laboratory based and require expertise to perform the assay [27,28].
In recent years, researchers are investigating the development of sensing devices in order to overcome the limitations of the standard CTX assays.
In 2009, Yun et al. [29] described label-free biosensor for the detection of CTX, based on electrochemical impedance spectroscopy (EIS) technique. In this work, a self-assembled monolayer (Dithiodipropionic acid) was deposited on the gold electrodes; streptavidin was then immobilized as a self-assembled monolayer on top of the Dithiodipropionic acid layer. After washing the electrodes to remove extra streptavidin, the electrodes were immersed in biotinylated antibody for 4 h, and then the electrodes were ready for the test.
The electrodes were dipped into the various concentrations of CTX solution, ranging from 0.2 to 10 μg/ml, for EIS measurements. Figure 1 illustrates the schematic representation of the proposed biosensor. A detection limit of 50 ng/ml was reported for the proposed immunosensor, but it was much higher than the reference range of CTX levels in human serum and urine.
Figure 1: Schematic demonstrating a label-free immunosensor for CTX measurement [29].
An optical fluorescence-based biosensor was developed by Kim et at. [30] for the detection of CTX in urine samples. This competitive assay relied on antibody-conjugated fluoro-microbeads in order to produce an optical signal. For this immunosensing, the surface of the optical probes was prepared by conjugating the antibody with the fluoro-microbeads.
A self-assembled monolayer (DTSP SAM) was used to immobilize PEG4-EKGPDP on the biosensing surface. A pre-mixed solution was prepared by mixing antibody-conjugated optical probes and different concentrations of CTX in urine samples. The pre-mixed solution was then applied to the sensing surface. The optical probes were involved in competition with the CTX molecules in the sample for binding to the immobilized PEG4-EKGPDP (Figure 2).
This immunoassay could detect CTX concentrations between 200 to 1400 ng/mmol covering the reference range required to diagnose osteoporosis. Using the proposed immunoassay, sensing could be achieved in 70 min, which was less than the time needed by conventional ELISA.
Later in 2015, Park et al. [31] reported a new method for the detection of CTX using a fluoro-microbeads guiding chip (FMGC) for simultaneous detection of serum and urinary CTX. The designed FMGC was made of three layers, as shown in Figure 3. The bottom layer was composed of a 180 × 800 μm2 gold sensing area on a silicon substrate.
The middle layer had four sensing areas, which were connected to the top layer via inter-connecting holes. The top layer had a sample injection inlet and four injection inlets. A fluidic control device (FCD) was designed and used to control the sample flow in channels separately. FCD enabled a particular and easy open-close action of FMGC channels.
In order to simultaneously measure the level of CTX in serum and urine using the developed FMGC, PEG4-EKGPDP and antibodies were immobilized on the sensing surface by using a self-assembled. The samples were then applied over the inlet for testing.
The signal was analysed by counting the fluorescent microbeads from the images which are already registered. The developed FMGC showed good correlation with ELISA and it required more than two hours to complete the detection and measurement procedure.
In 2016, an impedimetric immunosensing technique was proposed by Ramanathan et al. [32] utilizing gold-deposited carbon nanotube (CNT) electrode arrays, for the detection of CTX. Electrochemical impedance spectroscopy was implemented to detect the impedance changes happening on the surface of the gold-coated CNT electrodes.
The gold-coated electrodes were treated with avidin. Then the avidinated electrodes were immobilized with the biotinylated antibody. The sensor was then ready for the measurement. Different concentrations of CTX, ranging from 0.05 to 0.6 ng/ml were prepared. The sensor was immersed in the prepared solutions for one hour, and then it was rinsed in deionized water.
Finally, impedance measurements were made using the Gamry instrument. The schematic steps of biosensing surface preparation are given in Figure 4A. The developed biosensor could quantify CTX concentrations as low as 0.05 ng/ml but no real sample was tested using the proposed sensor.
Current research on development of biosensors for the measurement of CTX
We have developed a non-invasive, label-free and real-time biosensing technique for the early detection of bone loss by applying EIS method [33].
A silicon-based planar capacitive sensor with a dimension of 1 cm × 1 cm and gold interdigital electrodes, was used to perform the experiments (Figure 4B).
Figure 4b: Planar interdigital sensor [34].
In order to functionalize the sensor, the biosensing area (with a dimension of 2.5 mm × 2.5 mm) was first coated with streptavidin. A pre-mixed antibody-antigen solution was prepared by mixing different concentrations of antigen and antibodies.
Then, the antibody-antigen solution was applied on the sensing area for the detection of CTX. Finally, EIS measurement was performed using a high precision LCR meter. The schematic illustration for the sensing surface preparation is given in Figure 5.
Figure 5: Schematic diagram for the sensing surface preparation[33].
Various concentrations of CTX were measured using the proposed biosensing system. Figure 6 shows the cole-cole plot for four different concentrations of CTX in a frequency range of 40-100 Hz. Considerable changes could be observed in the diameter of the semicircles by changing the level of CTX in the test sample.
Figure 6: Nyquist (cole-cole) plot for different CTX concentrations[33].
Principal component analysis was employed to determine the most sensitive frequency, which was suggested 710 Hz showed the maximum changes in impedance. So the sensitivity of the biosensor was calculated from the following equation and the reference curve was plotted, which is given in Figure 7.
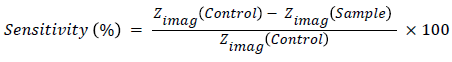
The reference curve was used to determine the concentration of CTX in the real serum samples. Two real serum samples from sheep blood were tested using the developed biosensing system. The results were compared with the results obtained from ELISA. The developed biosensor showed high correlation with ELISA.
Figure 7: The reference calibration curve for the sensitivity of the sensor vs. concentration at 710 Hz [33].
The developed biosensor was able to detect CTX concentrations between 0.147 ng/ml to 1.693 ng/ml, and it required approximately 2 h to perform the assay. However, there are still some limitations in using our developed biosensing device and all the biosensors were explained in the previous section. They are laboratory based and require technical expertise to perform the assay. Moreover, antibody immobilization steps are time-consuming and complicated. Also, natural antibodies have limited stability and are expensive which increases the cost of biosensing system. So preparing and using artificial antibodies can be a good solution to overcome the mentioned limitations.
In the last years, molecular imprinting polymers (MIPs) have been considered as a means to create artificial antibodies for different molecules [35-38]. Currently, we are working on the development of a MIP-based electrochemical biosensor for CTX detection and measurement [34]. Therefore, artificial recognition sites for CTX molecules were prepared by using precipitation polymerization technique.
Uptake kinetic and static adsorption studies were performed using a High-Performance Liquid Chromatography (HPLC) instrument to investigate the time required by the polymer to entrap the CTX molecules present in the sample and to determine the saturation level of MIP. A self-assembled monolayer of 3-aminopropyltrietoxysilane (APTES) was used to immobilize MIP on the surface of the interdigital gold electrodes. Figure 8 shows the MIP-coated sensor.
Figure 8: MIP coated interdigital sensor [34].
Different known concentrations of sample solutions ranging from 15 μg/ml to 45 μg/ml were prepared by dissolving CTX peptide in deionized water and tested using the proposed sensor. The EIS measurement was then performed using LCR meter and the obtained results were validated using HPLC. The Cole-Cole plot for different concentrations is shown in Figure 9.
Figure 9:Nyquist (Cole-Cole) plot for different concentration of CTX [34].
This is ongoing research, targeted to develop an inexpensive portable biosensor that can be used outside laboratories as a point-ofcare device for early detection of bone turnover. So far, we have worked on samples with high concentration and the results were encouraging for further investigations. We are now working on the preparation of polymer for very low concentrations to cover the reference clinical range of CTX in serum and urine.
This review has studied the available CTX assays and biosensors to measure the level of CTX in urine or serum samples. CTX is a sensitive biomarker of bone resorption that responds quickly to any change in bone metabolism. Despite the high sensitivity of available CTX assays such as ELISA, they are very costly and time-consuming. Recently, researchers have focused on the development of biosensors, which can overcome the limitations of ELISA and the available immunoassays. The recent researches are concentrating on the biosensors, which have great potential to simply and more frequently monitor bone metabolism.