Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2021)Volume 10, Issue 1
This work aims to study the antimicrobial activities of the essential oils of M. pulegium, P. chloranthus and T. algeriensis and to identify the antagonistic or synergistic impacts of their combinations. Antimicrobial activities were analyzed by disc diffusion and microdilution against six foodborne microbial strains (Staphylococcus aureus, Bacillus cereus, Escherichia coli, Salmonella enterica, Pseudomonas aeruginosa and Candida albicans). That's why, to predicate the optimal mix, we are using the Augmented Simplex Centroid Design. The chemical composition shows that the main compound of M. pulegium were pulegone (50.31%) and menthone (26.92%). For P. chloranthus EO, The major constituents were dillapiole (13.56%) and pregnane (8.47%) and the main products of T. algeriensis EO are Camphor (14.06%), β-Phellandrene (13.71%) and α-pinene (8.55%). The antimicrobial activity of the three studied EOs confirms the highest antibacterial activity of T. algeriensis and P. chloranthus EO against a lower antibacterial activity of M. pulegium EO. Moreover, the response surface analysis showed significant synergistic effects in some binary and ternary mixtures. The optimal mixture predicted against C. albicans corresponded to 12%, 35% and 53% of M. pulegium, P. chloranthus and T. algeriensis EOs, respectively. While the optimal mixture predicted against S. aureus was composed by P. chloranthus and T. algeriensis essential oils at 84% and 16%, respectively. Similarly, this binary mixture, between P. chloranthus and T. algeriensis, presented optimal antimicrobial activity against P. aeruginosa composed by essential oils at 20% and 80%, respectively. Generally, the combination between 19% M. pulegium, 41% P. chloranthus and 40% T. algeriensis consisting the optimal ternary mixture Eos. Our findings show that by the use of mixture design we can predict antibacterial interaction of essential oils. Therefore, it can be used as a natural antimicrobial agent and a food additives.
Mixture design; Antibacterial activity; Essential oils; Mentha pulegium; Pituranthos chloranthus; Thymus algeriensis
For many years there has been intense interest in essential oils as source of natural products. They have been screened for their potential uses as alternative remedies for the treatment of many infections and as natural food preservatives [1]. In fact, the essential oils represent an inexpensive source of natural antibacterial substances for use in pathogenic systems to prevent the growth of bacteria and extend the shelf life of the processed food [2]. Furthermore, enhancing food preservation and getting a balance between the sensory acceptability and antimicrobial efficacy is actually possible by the addition of small amounts of natural preservatives such as EOs. The Mentha pulegium L. (Lamiaceae) is widespread in America and thrives in Western, Southern and Central Europe, Asia, Iran, Arab countries and Ethiopia. Mentha pulegium is widely used in folk medicine in many cultures, aromatherapy and cosmetics [3]. The flowering aerial parts of this plant are traditionally used for their antimicrobial, expectorant, carminative and antispasmodics in the treatment of colds, bronchitis, tuberculosis, sinusitis, cholera, food poisoning, flatulence skin diseases, abortifacient and intestinal colic [4]. They strengthen the entire nervous system, stimulating diffusible and also a diffusible sedative; mint provides eminent services against nervousness and various nervous manifestations [5]. M. pulegium essential oil can be used as an antibiotic, a bio-insecticide and an organic food preservative, therefore a natural replacement for harmful synthesized chemicals. The essential oils of M. pulegium characterized by its richness in menthol, menthone and pulegone. The genus Pituranthos has more than twenty species, some of which are specific to North Africa [6]. Pituranthos species are often found in arid or desert regions. They are widespread in central and southern Tunisia. The genus Pituranthos has several therapeutic effects. Indeed, the species P. triradiatus and P. tartuosus, are used by the Bedouin population against stomach pains, intestinal parasites or as a menstrual regulator in women. Oils obtained from the stems and seeds of Pituranthos scoparius are widely used as a remedy against rheumatism and fever [7]. The species Pituranthos chloranthus is used, in poultices on the head, against the headache. In Morocco, the aerial parts are mixed with the ashes to flavour the meat [8]. It is also used in seasonings [9]. P. chloranthus stems have traditionally been used as straw by farmers to dry figs and grapes. In southern Tunisia, a tuft of P. chloranthus was traditionally suspended from the surface of water to disinfect underground rainwater storage tanks used for beverages [10]. There are many phytochemical studies on the Pituranthos chloranthus species, in particular, on its essential oils. Indeed, have demonstrated antimicrobial activity of P. chloranthus essential oils against Escherichia coli, Staphylococcus aureus and Enterococcus faecalis. The results of Yangui et al. clearly indicate the effective bactericidal and fungicidal action of essential oils of Tunisian P. chloranthus [7].
Thyme is considered as one of the most valuable spices and food preservatives in the food industry. T. algeriensis is the most pervasive North African species, endemic to Morocco, Tunisia, Algeria and Libya. It is is largely used in traditional medicine, in respiratory and digestive tube disorders and against abortion. Essential oils have been investigated in several studies where the scope was the chemical analysis of these compounds and their biological activities against several bacteria, yeast and fungi [11-13].
Goals of this work were to optimize the antibacterial and anticandidal Activity of the mixture of M. pulegium, P. chloranthus and T. algeriensis essential oils utilizing the Simplex- centroid Design.
Plant materials
The aerial parts of T. algeriensis and P. chloranthus were collected from Jabal Sagoufta, Qafsah (at the southwestern of Tunisia; latitude 34°30'0" (N); longitude 9°18'0" (E), altitude 567 m) in Avril. Plant populations of M. pulegium, was collected from Sousse, in central Tunisia, in May. The harvested plants were identified by Professor Fethia Harzallah-Skhiri (high institute of biotechnology of Monastir, Tunisia). The plant material was dried in the open air, in the dark and at room temperature for 2 weeks until constant weight. Moisture content was assessed by constant weight at 105°C and was 5.0 ± 0.5%, 4.47 ± 0.3 and 5.23 ± 0.41 of M. pulegium, P. chloranthus and T. algeriensis, respectively.
Extraction of the essential oil
Dried samples of the selected plants (100 g and 800 mL of water, ten times) were subjected to hydro-distillation for Clevenger in accordance with European Pharmacopoeia method [14].
The mixture was heated to boiling temperature and the liberated steams crossed up the column and passed out of the condenser in a liquid state. The obtained oils were separated completely from water without adding any solvent. The yield of the oil (based on dry plant weight) measured and stored in a freezer at 4°C in dark glass bottles until used.
In order to eliminate any trace of water in the Essential Oils (EO), the recovered distillate is stored in the freezer (-20°C) for 24 hours. The water phase is frozen and the essential oil is separated, measured and stored in freezer at 4°C in dark glass bottles until used EO yield (%) was measured using the following formula [15]:
EO yield (%) = Mass of EO obtained (g)/Mass of dry matter (g)× 100
Essential oil analysis
The analyze was carried out using GC 6890N and 5975B MS Agilent model, equipped with an Agilent Technologies capillary HP-5MS column (30 m × 0.25 mm i.d.) form of fused silica HP type -1 (0.25 μ film thickness) and an electron collision ionisation (70 eV ionization energy). The temperature of the injector starts from 35°C and increases 5°C/min to 250°C. The convayer gas was Helium used at 1 mL/min flow rate.
Identification of components was assigned by matching their mass spectra with Wiley and NIST library data standards of the main components and comparing their Kovats Retention Indices (KRI) with reference from the literature. The components concentrations were obtained by semi-quantification by peak area integration from GC peaks and by applying the correction factors [16].
The simplex centroid design method: 3-factors mixture design and triangular surface analyses were performed to optimize the antibacterial activity of mixture of three plants essential oils. The simplex-centroid design is composed with seven experiments. It is constructed to form a triangle with data points located at each corner, the three midpoints on each side, as well as the center (Figure 1).
Figure 1: Experimental points for the augmented Simplex-centroid design.
Mathematical models of mixing plans: The mathematical model applied to the mixing plans takes into account the fundamental constraint of mixing. The polynomials used have particularities that we will indicate.
First degree model: It is assumed that the variations in response are proportional to the composition of three-component mixture. Taking into account the fundamental constraint of mixtures:
x1 + x2 + x3 = 1
The model takes the following form:
y = β0 + β1x1 + β2x2 + β3x3
Second degree model: The second degree mathematical model includes first degree terms, quadratic terms and square terms. For a three-component mixture, we have:
Y = β1 x1+β2 x2+β3 x3+β12 x1 x2+β13 x1 x3 +β23 x2 x3
Third degree model: The simplified model, corresponding to a mixture of three components is as follows:
Y = β1 x1+β2 x2+β3 x3+β12 x1 x2+β13 x1 x3 +β23 x2 x3+ β123 x1 x2 x3
Where, y is the response function of experimental data, x1, x2 and x3 independent variables which correspond to the percentage of M. pulegium, P. chloranthus and T. algeriensis respectively, in the mixture.
βi= Yi
βij=4 Yij-2(Yi+ Yj)
βijk=27Yijk-12(Y12+Y13+Y23) + 3(Y1+Y2+Y3)
Preparation of mixtures of essential oils: The Simplex-centroid design has been used to prepare the mixtures of Eos. It composed of seven experiments with two replications at the center point. The obtained solutions were vortexed for about three min. Each solution has been diluted with DMSO (1:10 v/v) and vortexed again. The final volume of each eight mixtures was 6000 μL. Antibacterial and anti-candidal activity
Microorganisms: Antibacterial and antifungal activity was evaluated on different microorganisms provided by the Laboratory of Transmissible Diseases and Biological Active Substances (Faculty of Pharmacy of Monastir). Six microbial foodborne strains (5 bacteria and Candida) were used: Bacillus cereus (ATCC 6633), Salmonella enterica (CIP 8039), Escherichia coli (ATCC 8739), Staphylococcus aureus (ATCC 6538), Pseudomonas aeruginosa (ATCC 9027) and Candida albicans (ATCC 30031).
The micro-organism was grown in sterile broth medium for 24 h. Then 0.1 mL of each culture was mixed with 9.9 ml of fresh broth medium for 18 h.
Preparation of the culture medium
Preparation of Mueller Hinton medium: 38 grams of powder were added to 1 liter Distilled water and mixed thoroughly. The medium was boiling for 1-2 minutes to dissolve the constituents. Then, pH of the solution is adjusted to 7.3 and autoclaved at 120°C for 25 min.
Preparation of nutrient broth: 38 g of powder are dissolved in one liter of distilled water. The flask is placed in a boiling water bath until homogeneous, liquid, transparent solution is obtained. The solution is then poured into tubes, after which the latter have to be autoclaved at 120°C for 25 min.
Agar diffusion tests: In order to evaluate the antimicrobial activity of essential oils, we used the agar diffusion method of the National Committee for Clinical Laboratory Standards [17]. The bacteria and C. albicans were prepared from colonies of less than 24 h in Petri dishes. A single colony was mixed in 15 mL of the broth and then incubated for 3 to 5 hours at 35° C. then, Mueller- Hinton agar was inoculated with 100 μL of 106 CFU mL-1 bacterial cultures.
The plates were dried for 15 min. Then, Sterilized paper discs (6 mm) were impregnated with 10 μL of different EOs. After 30 min of diffusion at laboratory temperature, the Petri dishes were then incubated at 37°C for 18 to 24 hours. After the incubation, the antibacterial activity was expressed as the diameter of the inhibition zones (DIZ) produced and measured in mm unit. The tests were repeated twice, the Standard commercial antibiotics (Gentamicin) are used as a positive control.
The results were ranked as follows: not sensitive for zone diameters equal to 8 mm or below; sensitive for zone diameters between 8 and 14 mm, very sensitive for zone diameters between 14 and 20 mm and extremely sensitive for zone diameters equal or larger than 20 mm [18].
Minimum Inhibitory Concentration (MIC): The MIC is the lowest sample concentration capable to inhibit bacterial visible growth [19]. The MIC, MBC and MFC were determined according to the protocol [20]. Inocula of each bacterial strain were prepared in physiological water from a young culture. The eight samples of essential oils were dissolved in 10% dimethyl sulfoxide (DMSO). The suspensions were adjusted to a concentration of 106 bacteria/mL. MICs for the EOs were performed on a 96-wellplate:
• In column 1, we are deposed, 160 μL of Mueller-Hinton, 20 μL of the microbial suspension, 10 μL Gentamicin as positive control and 10 μL of resazurin as growth indicator, which is initially blue and turns pink in case of cell growth.
• In column 2, we are deposed, 170 μL of Mueller-Hinton, 20 μL of the microbial suspension and 10 μL of resazurin as negative control.
• In colomn 3, we are deposited a 200 μL of EOS mixture and dilution series of factor 2 was carried out while taking 100 μL of Column No. 3 and adding them in column No. 4 and so on to column 12. The last 100 μL of the wells of column 12 are discarded.
• From the third to the twelfth column, we added 70 μL culture medium 20 μL of the microbial suspension and 10 μL of resazurin.
• The plates were incubated at 37°C for 24 hours. The last well before the change of color in pink indicates the MIC.
Minimum Bactericidal Concentration (MBC)/Minimum Fungicide Concentration (MFC): The main steps in determining the MBC and MFC are as follows: 10 μL are taken from the well corresponding to the MIC and the one before. The specimens were then streaked (5 cm) onto the agar. The seeded Petri dish was subsequently incubated at 37°C for 24 hours. Finally, the absence of colonies of bacteria means that the corresponding concentration is that of the MBC. The CMB/CMI ratio allowed us to determine the bactericidal and bacteriostatic properties of the essential oil studied. When this ratio is greater than 4, the essential oil has a bacteriostatic power, and bactericidal when it is less than or equal to 4 [21].
Statistical analysis
The statistical significance of each equation is evaluated with Tukey's test at P<0.05. The ternary surface response diagram, polynomial model and principal component analysis were generated, was accomplished using JMP software.
Essential oil yields
Essential oils obtained from the aerial parts (stems and leaves) of M. pulegium, T. algeriensis and P. chloranthus yielded 2.4%, 1.2% and 0.89% (w/w), respectively.
M. pulegium could be considered as an appreciated source of EO and the obtained yields were higher than other ones collected from the north region of Tunisia (1.5%) and 1.84%. In Algeria, The extraction yield of EO from dried leaves of M. pulegium cultivated is about 1.45 ± 0.01%. However the result is similar to that of Morocco oil (2.33) and 2.7%. In Algeria, the average yield in leaves essential oils of T. algeriensis was significantly high (1.52-2.02%). The essential oil from the aerial parts of T. algeriensis, obtained by hydrodistillation, was obtained in a yield of 2.8 ± 0.2%, w/w [22]. The leaves yielded recorded for Tunisian T. algeriensis can be considered high compared to other Thymus species that are used industrially as a source of essential oils [12].
P. chloranthus EO yields from the fresh and dry herb collected from the Matmata’s mountainous chain in southern Tunisia are 1.6% and 0.9%, respectively [10].
Chemical composition
Twenty-five components accounting for 98.17% of the total amount of Tunisian M. pulegium EO were that represented approximately for Table 1. The EO is characterized with a high amount of oxygenated monoterpenes (82.78%). Main compound were pulegone (50.31%) and menthone (26.92%) followed by Palmitinic acid (7.32%). Therefore, pulegone is the oil chemotype. This has been confirmed by Abdelli et al. Hajlaoui et al. Bouchra, et al. They show that the percentage of this component varies between 25% to 92%. These results were in accordance with other studies. In fact, algerian study showed the richness of M. pulegium in pulegone (44.27%), menthone (19.05%) and piperitone (10.44%). Pulegone is also the major compound of Moroccan oil (69.8%) followed by piperitenone (3.1%). Moroccan study shows that The M. pulegium EO contains the pulegone (40.98%) and the menthone (21.164%) as major constituents. However, the major components of a fresh plant essential oil, were collected from the region of Beja (north of Tunisia) were menthol (39.2%), 1,8-cineole (17.1%), menthone (12.6%) and pulegone (11.7%). The chemical composition of Iranian oil is especially monoterpene: piperitone 38%, Menthone 39% [23]. Other compounds were poorly represented such as Isopulegone 2.89 and Steric acid 1.46 (Table 1). The total monoterpene hydrocarbons are 3.37%. The oxygenated sesquiterpenes and the sesquiterpene hydrocarbons are 1.69 and 1.43, respectively.
Table 1: Chemical composition, KI, retention time and percentage composition of the essential oils extracted from M. pulegium, P. chloranthus and T. algeriensis.
| No | Identification | KI | Rt (min) | Composition-Percentages (%) | ||
|---|---|---|---|---|---|---|
| M. pulegium | P. chloranthus | T. algeriensis | ||||
| 1 | Tricyclene | 926 | 6.04 | - | - | 0.34 |
| 2 | α-Thujene | 931 | 6.23 | - | 2.82 | 0.28 |
| 3 | α-Pinene | 940 | 6.52 | 0.96 | 7.43 | 8.55 |
| 4 | Camphene | 953 | 6.73 | - | 0.11 | 4.05 |
| 5 | Verbenene | 967 | 6.86 | - | - | 0.53 |
| 6 | β-Pinene | 971 | 7.44 | 0.2 | - | 4.74 |
| 7 | β-Phellandrene | 975 | 6.76 | - | - | - |
| 8 | Sabinene | 978 | 7.53 | - | 16.39 | - |
| 9 | β-Myrcene | 980 | 7.87 | 0.12 | 1.02 | 0.86 |
| 10 | α-Phellandrene | 1005 | 8.25 | - | 1.45 | 0.3 |
| 11 | 3-Carene | 1011 | 8.35 | 0.16 | 3.4 | - |
| 12 | α-Terpinene | 1016 | 8.6 | - | 1.45 | 1.22 |
| 13 | Cymene | 1020 | 8.83 | - | 1.4 | - |
| 14 | d-Limonene | 1031 | 8.88 | 0.79 | - | - |
| 15 | β-Phellandrene | 1026 | 8.92 | - | 1.16 | - |
| 16 | 1,8-Cineole | 1031 | 9.02 | - | - | 13.71 |
| 17 | α-Ocimene | 1035 | 9.21 | - | - | - |
| 18 | β-Ocimene | 1040 | 9.23 | - | 0.37 | 1.07 |
| 19 | γ-Terpinene | 1058 | 9.8 | - | 2.28 | 1.95 |
| 20 | 4-Thujanol | 1072 | 10.05 | - | 1.06 | 0.96 |
| 21 | α-terpinolene | 1081 | 10.61 | - | 0.79 | 1.08 |
| 22 | Linalool | 1098 | 11.03 | - | - | 2.68 |
| 23 | 1- terpineol | 1105 | 11.61 | - | 0.83 | - |
| 24 | Nopinone | 1122 | 12.02 | - | - | - |
| 25 | Camphor | 1136 | 12.27 | - | - | 14.06 |
| 26 | dl-Menthone | 1148 | 12.58 | 26.92 | 2.66 | - |
| 27 | Pinocarvone | 1157 | 12.73 | - | - | 0.97 |
| 28 | Borneol | 1161 | 12.94 | - | - | 1.25 |
| 29 | Isopulegone | 1167 | 13.15 | 2.89 | - | - |
| 30 | 4-Terpineol | 1171 | 13.31 | - | 7.32 | 4.22 |
| 31 | Cuminol | 1177 | 13.51 | - | - | |
| 32 | Menthol | 1180 | 13.52 | 0.42 | - | - |
| 33 | α-Terpineol | 1189 | 13.62 | - | 1 | 3.07 |
| 34 | Myrtenal | 1192 | 13.68 | - | - | - |
| 35 | Myrtenol | 1212 | 13.94 | - | - | 0.86 |
| 36 | Verbenone | 1228 | 14.1 | - | - | 1.3 |
| 37 | Carveol | 1235 | 14.44 | - | - | 0.92 |
| 38 | O-Methylthymol | 1244 | 14.74 | - | - | 0.28 |
| 39 | Cuminaldehyde | 1255 | 14.99 | - | - | 0.22 |
| 40 | Carvone | 1263 | 15.05 | - | - | 0.37 |
| 41 | Pulegone | 1271 | 15.17 | 50.31 | 3.95 | - |
| 42 | linalyl acetate | 1279 | 15.33 | - | - | 1.28 |
| 43 | piperitone | 1283 | 15.39 | 0.49 | - | - |
| 44 | Carane | 1291 | 15.88 | 1.05 | - | - |
| 45 | Camphane | 1293 | 15.88 | - | - | - |
| 46 | α-Fenchyl acetate | 1311 | 16.13 | - | - | 3.36 |
| 47 | Trans-Carane | 1342 | 16.75 | 0.76 | - | - |
| 48 | Carvacrol | 1356 | 16.95 | - | 0.31 | |
| 49 | 3-Terpinolenone | 1361 | 17.69 | 0.7 | - | - |
| 50 | α-Terpinene | 1365 | 17.82 | 0.38 | - | - |
| 51 | α-Ionone | 1367 | 17.83 | - | - | - |
| 52 | geranyl propanoate | 1371 | 18.23 | - | - | 0.2 |
| 53 | α-Copaene | 1378 | 18.49 | - | 0.1 | 0.15 |
| 54 | β-Bourbonene | 1384 | 18.72 | - | - | 0.26 |
| 55 | β-Elemene | 1391 | 18.91 | 0.18 | - | 0.15 |
| 56 | α-Gurjunene | 1415 | 19.33 | - | - | 0.27 |
| 57 | Methyleugenol | 1423 | 19.35 | - | 1.11 | - |
| 58 | β-Caryophyllene | 1442 | 19.6 | 0.15 | 0.31 | 0.35 |
| 59 | α-Bergamotene | 1449 | 20 | - | 0.12 | - |
| 60 | α –humulene | 1451 | 20.46 | 0.34 | - | 0.04 |
| 61 | β-Farnesene | 1443 | 20.53 | - | 0.24 | - |
| 62 | Aromadendrene | 1456 | 20.63 | - | - | 0.27 |
| 63 | Germacrene-D | 1468 | 21.17 | - | 0.64 | 0.43 |
| 64 | Bicyclogermacrene | 1471 | 21.5 | - | - | 0.45 |
| 65 | Mint furanone | 1480 | 21.66 | 0.75 | - | |
| 66 | α-Farnesene | 1505 | 21.81 | - | 0.2 | - |
| 67 | α-Amorphene | 1512 | 21.94 | 0.25 | - | 0.22 |
| 68 | Δ-Cadinene | 1525 | 22.15 | - | - | 0.56 |
| 69 | L-calamenene | 1527 | 22.16 | 0.68 | - | - |
| 70 | Myristicin | 1532 | 22.39 | - | 2.4 | - |
| 71 | α-Calacorene | 1539 | 22.62 | - | - | 0.06 |
| 72 | Hedycaryol | 1541 | 22.85 | - | - | - |
| 73 | Elemol | 1546 | 22.87 | - | - | 0.65 |
| 74 | Elemicin | 1554 | 23.08 | - | 0.4 | - |
| 75 | Palustrol | 1562 | 23.24 | - | - | 0.15 |
| 76 | Caryophyllene oxide | 1576 | 23.59 | 0.34 | - | 2.23 |
| 77 | Spathulenol | 1585 | 23.6 | - | 0.89 | |
| 78 | Veridiflorol | 1614 | 23.9 | - | - | 3.31 |
| 79 | Globulol | 1623 | 24.09 | - | - | 0.38 |
| 80 | Adipol | 1643 | 24.53 | - | - | 0.14 |
| 81 | Dillapiole | 1649 | 24.55 | 0.12 | 13.56 | - |
| 82 | Cadina-1,4-diene | 1658 | 24.65 | 0.43 | - | - |
| 83 | β-Maaliene | 1672 | 24.72 | - | - | 0.13 |
| 84 | β-Eudesmol | 1693 | 25.2 | - | 1.47 | 0.47 |
| 85 | t-Muurolol | 1703 | 25.25 | - | - | 0.21 |
| 86 | β-Himachalene | 1716 | 25.38 | - | - | 0.73 |
| 87 | Azulol | 1736 | 25.69 | - | - | 0.24 |
| 88 | 14-Norcadin-5-en-4-one | 1771 | 26 | - | - | 0.16 |
| 89 | Butylphthalide | 1821 | 27.24 | - | 0.37 | - |
| 90 | Palmitinic acid | 1876 | 27.92 | 7.32 | - | 1.39 |
| 91 | 2-Pentadecanone | 1902 | 29.21 | - | - | |
| 92 | Steric acid | 1928 | 30.73 | 1.46 | - | - |
| 93 | Dodecanamide | 1943 | 31.93 | - | ||
| 94 | 13-Epimanoyl oxide | 1954 | 32.11 | - | - | - |
| 95 | Manoyl oxide | 1968 | 32.17 | - | 0.58 | - |
| 96 | Anthracene | 2015 | 32.72 | - | - | - |
| 97 | Dehydroabietane | 2026 | 33.08 | - | 0.33 | - |
| 98 | Oleic acid | 2093 | 34.17 | - | - | 0.4 |
| 99 | Hexadecane | 2112 | 34.31 | - | - | |
| 100 | Steric acid | 2134 | 34.41 | - | - | 0.74 |
| 101 | Tetracosane | 2226 | 36.17 | - | - | |
| (17E)-Pregna-5,17-dien-3-ol | 2251 | 36.8 | - | - | - | |
| 102 | Hexacosane | 2356 | 38.41 | - | - | - |
| 103 | γ.-Sitosterol | 2485 | 41.51 | - | - | - |
| 104 | Hentriacontane | 2524 | 44.44 | - | - | - |
| 105 | Pentyl acetate | 2562 | 45.17 | - | - | - |
| 106 | 14B-Pregnane | 2584 | 46.66 | - | 8.47 | 0.19 |
| 107 | Cetyl vinyl ether | 2588 | 46.67 | - | - | - |
| 108 | Nonacosane | 2896 | 46. 96 | - | - | 0.15 |
| 109 | Hexatriacontane | 2902 | 46.97 | - | - | - |
| 110 | Capnellene | 2913 | 47 | - | 2.67 | - |
| 111 | Clionasterol | 2928 | 47.24 | - | - | |
| 112 | Sitostenone | 2934 | 47.39 | - | - | - |
| 113 | Spongesterol | 2938 | 47.4 | - | 2.27 | |
| 114 | Friedelin | 2972 | 47.56 | - | - | - |
| 115 | 3-Ethylstyrene | 2981 | 47.59 | - | - | - |
| 116 | Cycloartenol | 2993 | 47.64 | - | 1.44 | - |
| 117 | Epifriedelinol | 3134 | 48.25 | - | - | - |
| 118 | Stigmastan-3,5-dien | 3165 | 48.64 | - | - | 0.11 |
| Total monoterpene hydrocarbons | 3.37 | 40.07 | 24.67 | |||
| Total oxygenated monoterpenes | 82.78 | 17.13 | 46.15 | |||
| Total sesquiterpene hydrocarbons | 1.43 | 9.66 | 5.17 | |||
| Total Oxygenated sesquiterpenes | 1.69 | 8.26 | 10.85 | |||
| Total identified compounds | 98.17 | 94.77 | 89.11 | |||
For P. chloranthus EO, The major constituents were dillapiole (13.56%) and pregnane (8.47%). The essential oil contains mainly monoterpene hydrocarbons (40.07%) and oxygenated monoterpenes (17.13%). In literature, there is difference on composition between P. chloranthus oils. This suggests that there are different chemotypes of P. chloranthus in Tunisia. For exemple, the main constituents of the EO obtained from P. chloranthus collected in Southern Tunisia were found to be α-pinene (32.5%), β-phellandrene (13.9%) and α-phellandrene (7.8%). EO of P. chloranthus collected from Sfax present also main constituent (terpinen-4-ol (30.3%)) which is different from other regions in Tunisia and other countries. A comparison between these studies showed the variability of volatile compound. The variation in the obtained results is certainly attributed to the difference in geographical locations and growth conditions that affect the chemical composition of Eos.
At last, according to the results on Table 1, the constituents of the essential oil of T. algeriensis is more than 60 compounds with an overall percentage of 89.11%. The chemical composition is particular and characteristic of the essential oil. This table shows that the essential oil contains mainly oxygenated compound (46.15%) and monoterpene hydrocarbons (24.67%).
The main products are Camphor (14.06%), β-Phellandrene (13.71%) and α-pinene (8.55%). Similarly, Mehalaine et al. identified camphor (13.62%) as the main constituent of T. algeriensis EO. However, the results in the literature indicate that the essential oils of Thymus algeriensis collected in Tunisia, Algeria and Morocco have a high level of carvacrol. In fact, the main compound of essential oils of T. algeriensis cutivated from three Tunisian region (Korbous, Jdidi Jebel Montain and Hammem Sousse) were caryophylleneoxide (18.5-25.3%), veridiflorol (tr-39.7%), α-pinene (2.7-15.2%), 1,8-cineole (1.2-12.8%), p-eugenol (tr-15.8%), geraniol (tr-7.1%). In Algeria, Salah Bendjabeur (2018) showed that the main constituents were carvacrol (43.2%), γ-terpinene (14.8%), p-cymene (18.7%), and thymol (5.6%). In Marroco, The essential oil was characterized by high amounts of Geranyl acetate (80.8%). The other major components were Geraniol (7.3%) and trans- Caryophyllene (2.4%). But, work of Hamdani showed that borneol (28%), camphene (20.9%) and camphor (15.7%) were the major compounds. T. algeriensis EO collected from Libya thymol was the main constituent (38.5%) fol-lowed by p-cymene, terpinene, bornyl acetate and borneol (8.9%, 7.1%, 7.0% and 6.0%, respectively).
Evaluation of antibacterial activity
Single antibacterial effect: The study of the antimicrobial activity of EO by the disc diffusion method shows that, with the exception of P. aeruginosa, M. pulegium EO has a bacteriostatic activity against all the strains tested. Table 2 shows that Gram-positive; S. aureus (14 ± 1 mm) and B. subtilis (13 ± 2 mm) bacteria are very sensitive to essential oils. For Gram-negative bacteria and Yeasts, M. pulegium's EO has significant inhibitory effects against E. coli (11 ± 0.8 mm), S. enteritidis (10 ± 1.5 mm) and Candida albicans (11 ± 1.5 mm).
Table 2: Antimicrobial and anticandidal activity of Mentha pulegium, Pituranthos chloranthus and Thymus algeriensis essential oils.
| Microbial strains | M. pulegium | P. chloranthus | T. algeriensis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Inhibition zone (mm) | MIC (µL/mL) | MBC (µL/mL) | Inhibition zone (mm) | MIC (µL/mL) | MBC (µL/mL) | Inhibition zone (mm) | MIC (µL/mL) | MBC (µL/mL) | |
| Bacteria Gram+ | |||||||||
| S. aureus | 11 ± 1 | 20 | 40 | 19 ± 1.75 | 2.5 | 5 | 15 ± 2 | 5 | 5 |
| B. cereus | 13 ± 2 | 2.5 | 5 | 14 ± 1.25 | 1.25 | 5 | 12 ± 1.5 | 2.5 | 5 |
| Bacteria Gram- | |||||||||
| E. coli | 11 ± 0.8 | 5 | 10 | 16 ± 0.75 | 2.5 | 2.5 | 13 ± 1.25 | 1.25 | 5 |
| S. enterica | 10 ± 1.5 | 5 | 5 | 12 ± 1.2 | 5 | 5 | 11 ± 1.4 | 5 | 5 |
| P. aeruginosa | <8 | - | - | 9 ± 1 | 20 | 40 | 9 ± 1 | 10 | 20 |
| Yeasts | |||||||||
| C. albicans | 11 ± 1.5 | 20 | 20 | 17 ± 1.2 | 2.5 | 2.5 | 19 ± 1.5 | 0.625 | 2.5 |
Means values ± SD of triplicate determination
As shown in Table 2, CMI values confirm those obtained previously by Aromatogram method. The strains tested are sensitive to M. pulegium EO at different concentrations from 2.5 to 20 μL/mL. The lowest MIC value (2.5 μL/mL) was observed in B. cereus.
Similarly, Abdelli et al. show that M. pulegium's EO is active against bacteria Gram négative such as, Bordetella bronchiseptica, Escherichia coli, Pasteurella multocida, Salmonella enteritidis and S. gallinarum pullorum. They show also that bacteria Gram positive are extremely sensitive to this EO in particular S. aureus (23 ± 1 mm) and B. subtilis (24 ± 0 mm). Another study carried out by Ait-Ouazzou et al. indicate that M. pulegium's EO inhibited the growth of E. coli by causing an inhibition zone with a diameter of 12.6 ± 0.5 mm. in like manner, the results found by Hajlaoui et al. showed that M. pulegium EO has a strong anti-microbial activities in particular against Gram-positive bacteria with inhibition zones in the order of 10-31 mm [24]. However, the study by Boukhatem et al. (2014) shows that yeasts are more resistant than the pathogenic bacteria tested [25].
The potential antimicrobial activity of this M. pulegium EO is explained with the high level of oxygenated monoterpenes (82.78%) in particular pulegone and menthone. However, other trace components could increase the antimicrobial activity. In addition to that, it is possible to have a synergistic and antagonistic interaction between the components. Several researchers have studied the mode of action of EO. They have shown that antimicrobial activity is caused by the action of terpenes in enzymatic systems related to energy production and in the synthesis of structural components of microbial cells [26]. In fact, EO crosses the cell membrane, interacting with enzymes and proteins in the H+/ATPase pumping membrane, producing a proton flow that induces cell death. In addition, terpenes can affect the permeability and other functions of cell membranes. Indeed, EO crosses cell membranes, enters the cell and interacts with critical intracellular sites [27].
The results of the evaluation of the antimicrobial activities of P. chloranthus and T. algeriensis EOs are presented in Table 2. This result exhibited a potent antibacterial activity against all tested bacteria and fungi. They indicate that Gram negative bacteria were more susceptible to the antimicrobial properties of essential oils (inhibition zones in the order of 9-14 mm) than Gram positive bacteria and yeasts (inhibition zones in the order of 14-19 mm).
Accurately, MICs/MFC and MBSs values for P. chloranthus essential oil against S. aureus, B. cereus, E. coli, S. enterica and C. albicans colonies ranged from 1.25 μL/mL to 5 μL/mL; for T. algeriensis essential oil these values were varied from 0.625 μL/mL to 5 μL/ mL. However, P. aeruginosa were found to be less sensitive to the tested EOs and tend to display higher MIC values (20 μL/mL and 10 μL/mL for P. chloranthus and T. algeriensis Eos, respectivily) and higher MBC (ranged from 40 to 20 μL/mL). The activity of essential oils correlates with their chemical function. Indeed, the biological activity of an essential oil is related to its chemical composition, the functional groups of the majority compound. Minority compounds also play an important role in the activity of essential oils and appear to act in synergy with the main compounds) [28].
The high antimicrobial activity P. chloranthus and T. algeriensis EOs is explained by the presence of high amounts of oxygenated monoterpenes (17.13 and 46.15%, respectively) and monoterpene hydrocarbons (40.07 and 24.67, respectivily). Indeed, Cox et al. show that monoterpenes are capable of affecting cellular integrity, leading to inhibition of respiration and altered permeability [29].
Similarly, the results of Yangui et al. indicate the effective bactericidal and fungicidal action of essential oils of Tunisian P. chloranthus where the antimicrobial effect against four microorganisms : P. aeruginosa, E. coli, S. aureus and E. hirae, as well as on yeasts: C. albicans and Aspergillus niger. In addition, Neffati, in 2009 reported good activity bacteriostatic of the essential oil of P. chloranthus against all Gram-positive bacteria studied, in particular S. aureus and L. monocytogene [30].
The antibacterial and antifungal activities of T. algeriensis EOs have studied by Ali, which is in agreement with our obtained results. They showed moderate antibacterial and antifungal activities with growth zone ranged from 13.6 to19.4 mm. The highest MIC value was detected against P. aeruginosa, while the lowest was observed against B. cereus. A moderate inhibitory concentration was against L. monocytogenes and E. coli (MICs=3.5-5 μL/mL). In conclusion, essential oil is a complex mixture of different chemical components, thus it is difficult to reduce the antimicrobial effect of the total essential oil to one or more active ingredients. In addition, other work shows that minor components, as well as a possible interaction between substances, may affect antimicrobial activities. The high antibacterial and antifungal activities of Eos suggests the possibility of using this plant as a natural antimicrobial preservative in the food and pharmaceutical systems.
Figure 3 shows ternary contour plots of the responses (MICs) for each studied bacteria. Optimal design regions for the antibacterial effect of EOs mixture are the colourless areas. Figure 3b indicates that the CMI contour values increased toward the P. chloranthus where minimum CMI contour could be seen. Figure 3e and Figure 3f indicate that the CMI contour values increased toward T. algeriensis. Moreover, Figure 3d shows that the CMI contour values increased toward M. pulegium; however, for E. coli (Figure 3c) strain the lowest CMI values were at T. algeriensis-P. chloranthus edge.
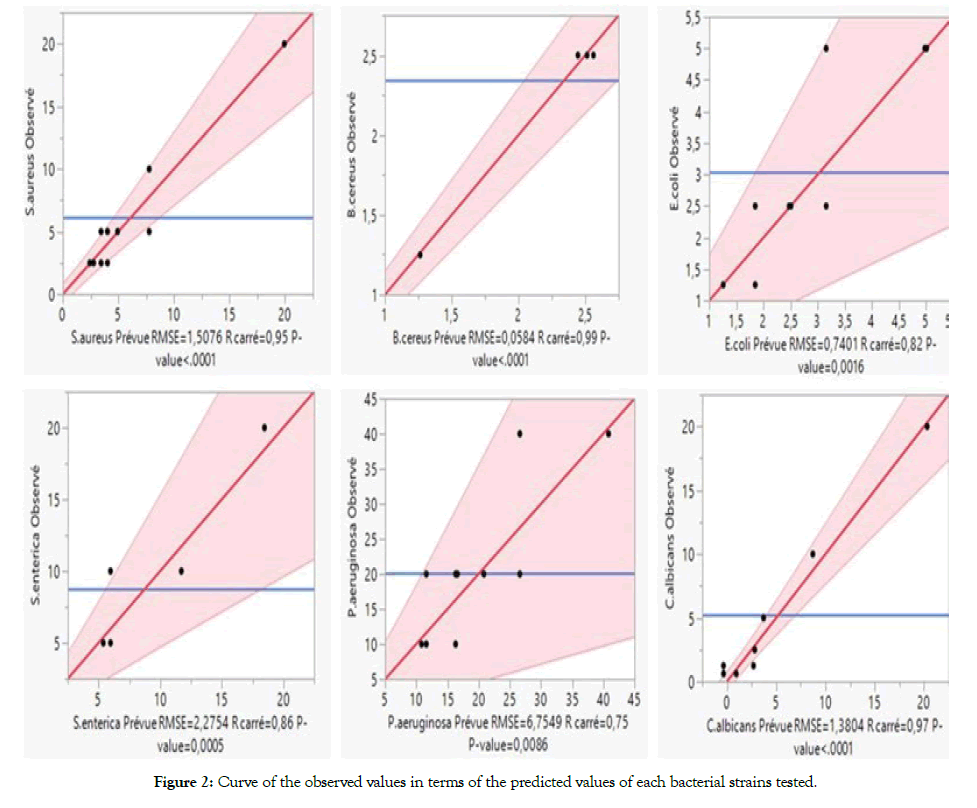
Figure 2: Curve of the observed values in terms of the predicted values of each bacterial strains tested.
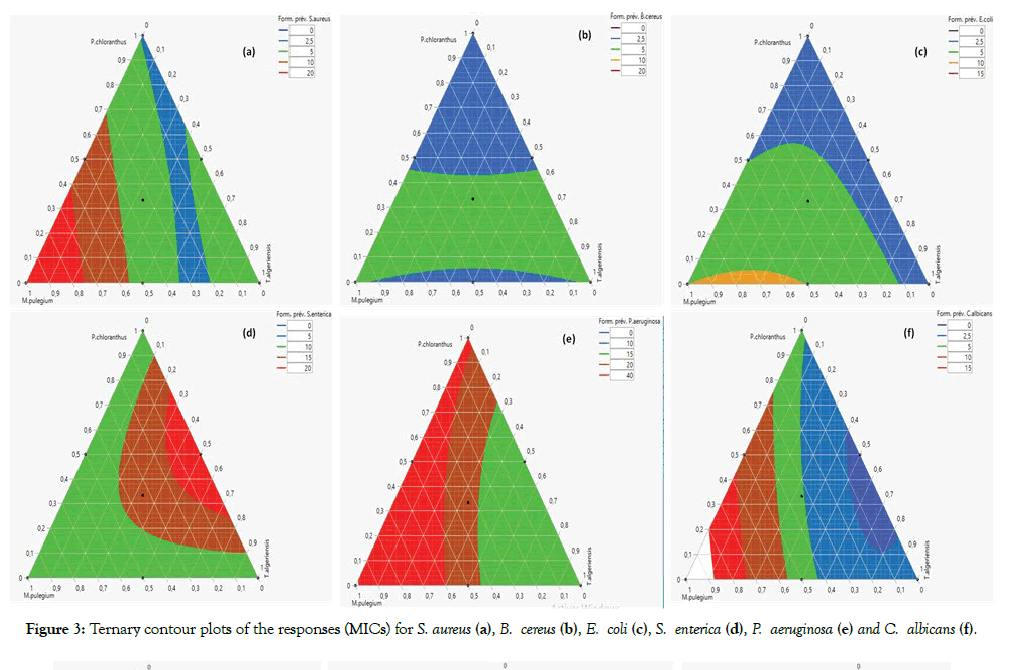
Figure 3: Ternary contour plots of the responses (MICs) for S. aureus (a), B. cereus (b), E. coli (c), S. enterica (d), P. aeruginosa (e) and C. albicans (f).
Similarly, the isoresponses curves (Figure 4) shows the compromise areas between the components. In fact, the colourless areas in the Figures 4a, 4b, 4e and 4f indicate CMI values less than 2.5, Figure 4d the isoreponse curves of CMI values less than 5 delimit the colourless zone. Whereas, the colourless areas in the Figure 4c indicate CMI values less than 15. Besides, the exact optimal combination with a percentage of compromise was found by use of the "Desirability" function. The exact optimum setting has shown in Figure 5. The minimum CMI values against E. coli (Figure 5a), B. cereus (Figure 5b) and S. enterica (Figure 5c) are obtained with a desirability of 100% T. algeriensis, 100% P. chloranthus and 100% M. pulegium, respectively whereas, the precise proportions of binary EOs mixtures leading to the optimal antibacterial activity against S. aureus (Figure 5d) and P. aeruginosa (Figure 5e) were 16% T. algeriensis-84% P. chloranthus and 80% T. algeriensis-20% P. chloranthus, respectively. The optimal anticandidal was obtained by realizing ternary EO mixture consisting 12% M. pulegium, 35% P. chloranthus and 53% T. algeriensis (Figure 5f).
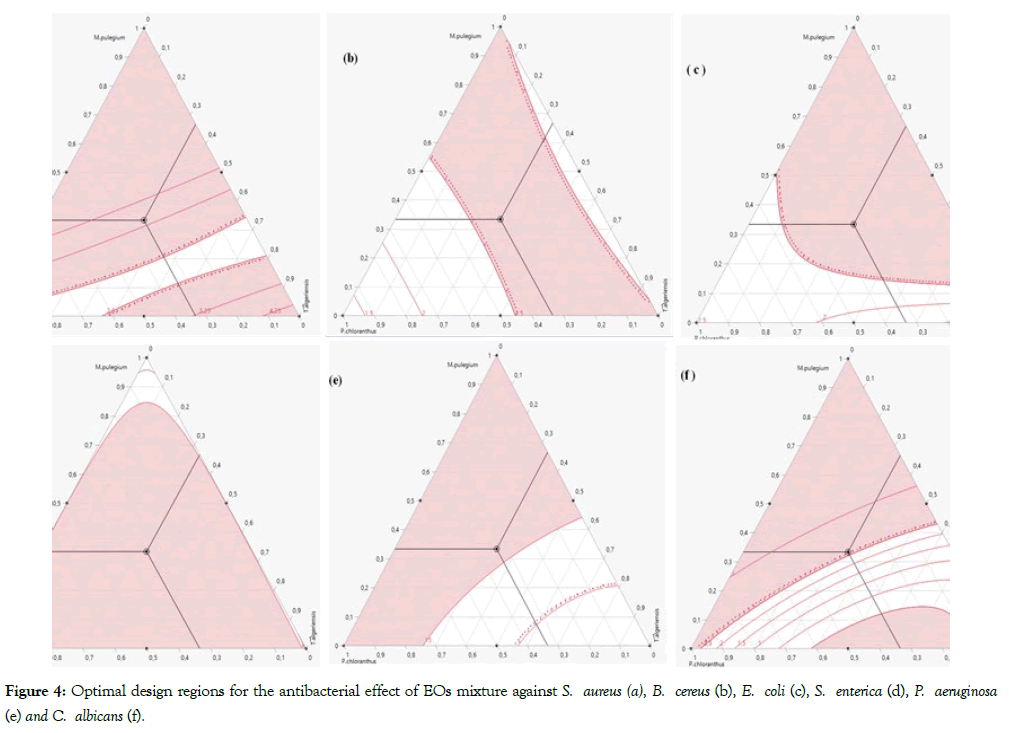
Figure 4: Optimal design regions for the antibacterial effect of EOs mixture against S. aureus (a), B. cereus (b), E. coli (c), S. enterica (d), P. aeruginosa (e) and C. albicans (f).
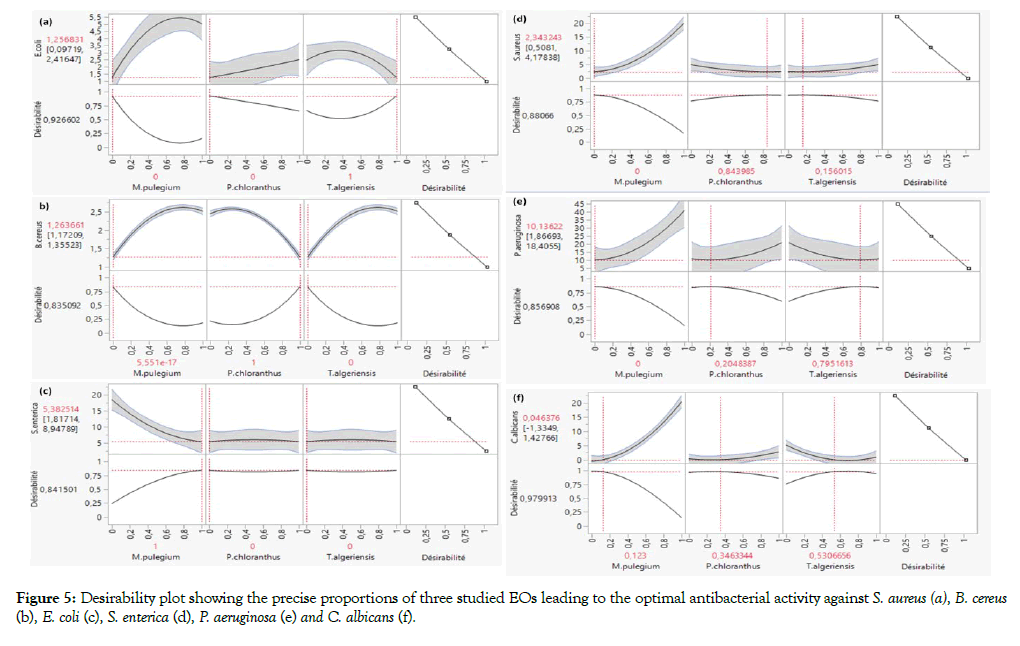
Figure 5: Desirability plot showing the precise proportions of three studied EOs leading to the optimal antibacterial activity against S. aureus (a), B. cereus (b), E. coli (c), S. enterica (d), P. aeruginosa (e) and C. albicans (f).
And to finish, Figure 6 shows the plot desirability of the precise proportions of three studied EOs leading to the desired MIC value equal to 2.5 against the strain S. aureus (Figure 6d), B. cereus (Figure 6b), E. coli (Figure 6a); less than 15 for S. enterica (Figure 6c), P. aeruginosa (Figure 6e) and less than 1 against C. albicans (Figure 6f). Thus, the point of the optimal mixture consisting 19% M. pulegium, 41% P. chloranthus and 40% T. algeriensis. These results have suggested a possible synergistic or additive effect between these three Eos. The point on the optimal mixture was used to confirm the validity of the postulated model and there is any significant difference between the predicted and experimental responses. Hence, the optimal mixture should be considered as a potential alternative for control of food safety.
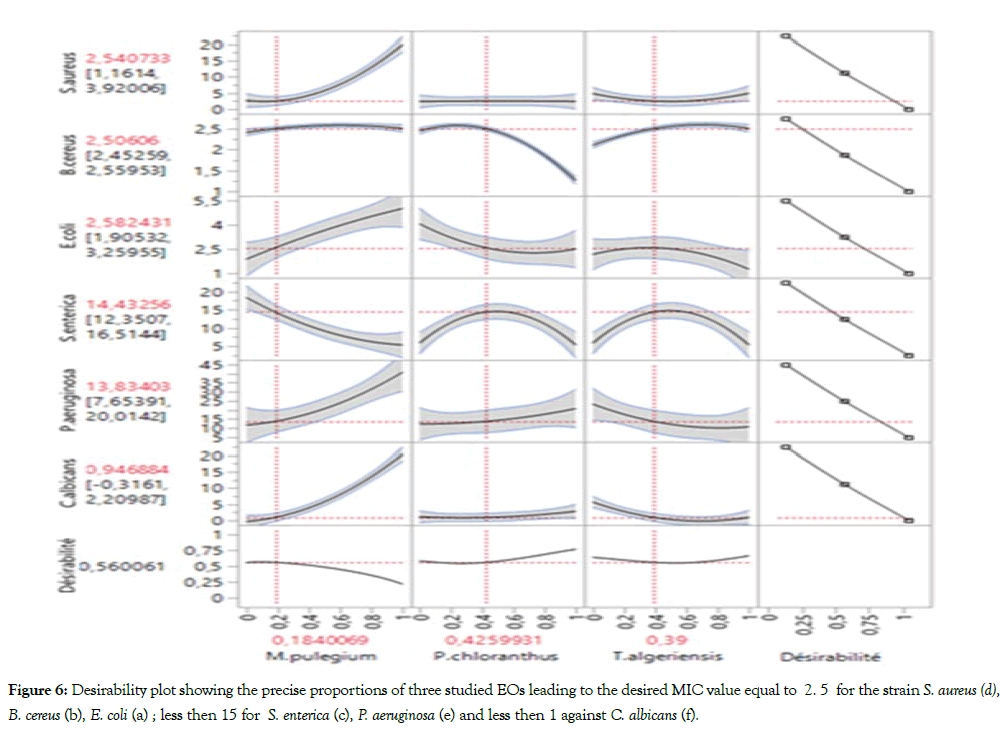
Figure 6: Desirability plot showing the precise proportions of three studied EOs leading to the desired MIC value equal to 2. 5 for the strain S. aureus (d), B. cereus (b), E. coli (a) ; less then 15 for S. enterica (c), P. aeruginosa (e) and less then 1 against C. albicans (f).
Optimization of the anti-bacterial and anti-candidal effect: In other to predict the antibacterial combined effect of M. pulegium, T. algeriensis and P. chloranthus essential oils and define the optimal mixture, the augmented simplex-centroid mixture design was chosen.
The mixtures design of the three studied EOs and the experimental responses (MICs) obtained on each studied strain are listed in Table 3. Therefore, the JMP software coefficient estimation section shows the values of the model coefficients (Figure 7). The effect is statistically significant, when the p-value was less than 5%. Generally, the effects of the pure components are significant, except T. algeriensis EO against C. albicans. as shown in Figures 7a-7f with p-value=0.352. Furthermore, negative sign of a coefficient shows the response decrease while the factors values increase while the positive sign indicates that the ability of a factor to increase the response variable. As the aim of this study is to minimize the MIC values, a negative sign of the coefficient is targeted to increase the antibacterial effect. In fact, the interaction between mixture components can produce four types of outcomes: indifferent, additive, antagonistic and synergistic outcomes. Figure 7 showed significant synergistic or additive effects in some binary mixtures, in particular M. pulegium-T. algeriensis and M. pulegium-P. chloranthus against S. aureus (a), and C. albicans (f), whereas the binary mixture T. algeriensis-P. chloranthus is produced indifferent or antagonistic outcomes. The indifferent effect is against S. aureus (a), E. coli (c), P. aeruginosa (e) and C. albicans (f); the antagonistic outcome is against B. cereus (b) and S. enterica (d).
Table 3: Original components of the design matrix and experimental responses (MICs) obtained for each bacteria.
| Original components of the design matrix | Experimental responses (MICs) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| M. pulegium | P. chloranthus | T. algeriensis | S. aureus | B. cereus | E. coli | S. enterica | P. aeruginosa | C. albicans | |
| 1 | 0.000 | 0.000 | 1.000 | 5.000 | 2.500 | 1.250 | 5.000 | 10.000 | 0.625 |
| 2 | 0.000 | 0.000 | 1.000 | 5.000 | 2.500 | 1.250 | 5.000 | 10.000 | 0.625 |
| 3 | 0.000 | 0.500 | 0.500 | 2.500 | 2.500 | 2.500 | 20.000 | 10.000 | 1.25 |
| 4 | 0.000 | 0.500 | 0.500 | 2.500 | 2.500 | 1.250 | 20.000 | 20.000 | 0.625 |
| 5 | 0.000 | 1.000 | 0.000 | 2.500 | 1.250 | 2.500 | 5.000 | 20.000 | 2.5 |
| 6 | 0.000 | 1.000 | 0.000 | 2.500 | 1.250 | 2.500 | 5.000 | 20.000 | 2.5 |
| 7 | 0.333 | 0.333 | 0.333 | 5.000 | 2.500 | 10.000 | 10.000 | 10.000 | 1.25 |
| 8 | 0.333 | 0.333 | 0.333 | 5.000 | 2.500 | 10.000 | 10.000 | 10.000 | 1.25 |
| 9 | 0.333 | 0.333 | 0.333 | 2.500 | 2.500 | 10.000 | 10.000 | 10.000 | 1.25 |
| 10 | 0.333 | 0.333 | 0.333 | 2.500 | 2.500 | 10.000 | 10.000 | 20.000 | 1.25 |
| 11 | 0.500 | 0.000 | 0.500 | 2.500 | 2.500 | 10.000 | 10.000 | 20.000 | 5.000 |
| 12 | 0.500 | 0.000 | 0.500 | 5.000 | 2.500 | 5.000 | 5.000 | 20.000 | 5.000 |
| 13 | 0.500 | 0.500 | 0.000 | 10.000 | 2.500 | 2.500 | 10.000 | 20.000 | 10.000 |
| 14 | 0.500 | 0.500 | 0.000 | 5.000 | 2.500 | 5.000 | 5.000 | 40.000 | 10.000 |
| 15 | 1.000 | 0.000 | 0.000 | 20.000 | 2.500 | 5.000 | 5.000 | 40.000 | 20.000 |
| 16 | 1.000 | 0.000 | 0.000 | 20.000 | 2.500 | 5.000 | 5.000 | 40.000 | 20.000 |
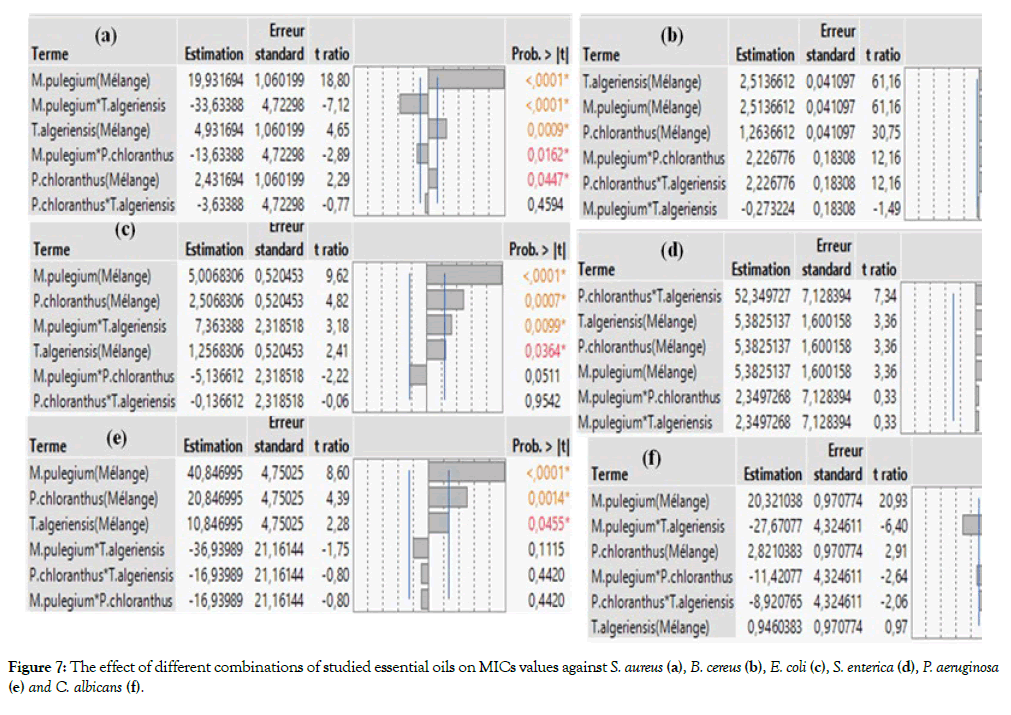
Figure 7: The effect of different combinations of studied essential oils on MICs values against S. aureus (a), B. cereus (b), E. coli (c), S. enterica (d), P. aeruginosa (e) and C. albicans (f).
Besides, Table 4 predicted the special cubic models for the experimental responses (MICs) from EOs mixtures. They describe the relationship between the MICs values and the fraction of each EOs. The prediction purpose for the R2 values is higher than 0.74 which considered adequate [30]. The relationship between the observed and predicted values (Figure 2) predicted a linear curve. These results were confirmed a good agreement between the predicted and the experimental values.
Table 4: Predicted models of the responses (MICs) obtained for each bacteria.
| Bacteria | Predicted models |
|---|---|
| Staphylococcus aureus | Y=19.94 X1+ 2.43 X2 + 4.9 X3-13.63 X1 X2 – 33.63 X1 X3 -3.63 X2 X3 |
| Bacillus cereus | Y=2.5 X1+ 1.26 X2+ 2.5 X3+ 2.22 X1 X2- 0.27 X1 X3 + 2.22 X2 X3 |
| Escherichia coli | Y=5 X1+ 2.5X2+ 1.25 X3-5.13 X1 X2+7.37X1 X3 -0.13X2 X3 |
| Salmonella enterica | Y= 5.38 X1+ 5.38 X2+ 5.38 X3+ 2.34 X1 X2+ 2.34 X1 X3 + 52 X2 X3 |
| Pseudomonas aeruginosa | Y=40.84 X1+ 20.84 X2 + 10.84 X3 - 16.93 X1 X2 - 36.93 X1 X3 - 16.93 X2 X3 |
| Candida albicans | Y=20.32 X1 + 2.82 X2 + 0.94 X3 – 11.42 X1 X2- 27.67 X1 X3 – 8.92 X2 X3 |
In conclusion, these results showed that T. algeriensis, M. pulegium, P. chloranthus EOs alone or combined are effective against the six microbial foodborne strains (B. cereus, S. enterica, E. coli, S. aureus, P. aeruginosa and C. albicans). The screening of antimicrobial activity of the three studied EOs allowed us, firstly, to confirm the highest antibacterial activity of T. algeriensis EO followed by P. chloranthus EO against E. coli, P. aeruginosa and C. albicans and the highest antibacterial activity of P. chloranthus EO followed by T. algeriensis EO against S. aureus and B. cereus; however, results show the lowest antibacterial activity of M. pulegium against all studied strains. Secondly, we observed a great potential of M. pulegium EO in combined with T. algeriensis or P. chloranthus against S. aureus, and C. albicans. This result was explained by a possible synergistic or additive of double and triple combinations of EOs constituents. Especially, the combination between 19% M. pulegium, 41% P. chloranthus and 40% T. algeriensis consisting the optimal ternary mixture Eos. These results indicate the possible use of the essential oils on food system as antimicrobial agents. In this way, these results should be a promising approach and an interesting for the optimization of food preservation, considering both sensory quality of food and economic aspects.
We thank Laboratory of Transmissible Diseases and Biologically Active Substances, the Laboratory of Pharmacognosy at Faculty of Pharmacy, and the Laboratory of Chemistry of Natural Substances, Faculty of Sciences of Sfax, for helping and their internship, especially the quality service.
Citation: Souiy Z, Elaissi A, Jlassi I, Sghair W, Allouch N, Mastouri M, et al. (2020) Application of Simplex-Centroid Design Methodologies to Optimize the Anti-bacterial and Anti-candidal Activity of the Mixture of Mentha pulegium, Pituranthos chloranthus and Thymus algeriensis Essential Oils. Med Aromat Plants (Los Angeles) 9: 365. doi: 10.35248/2167-0412.20.9.365
Received: 28-Sep-2020 Accepted: 30-Dec-2020 Published: 07-Jan-2021 , DOI: 10.35248/2167-0412.21.10.365
Copyright: © 2020 Souiy Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.