Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2020)Volume 9, Issue 6
Introduction: Clinacanthus nutans (CN) is a medicinal herb that is traditionally used in Southeast Asia as a treatment for disease and prevention of cancer.
Objective: The goal of this study was to investigate the effects of different drying processes on properties of CN.
Methods: Air dried (ADDCM) and oven dried (ODDCM) leaves were extracted using the dichloromethane (DCM) Soxhlet extraction method. Antioxidant activity of the extracts was measured using the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) scavenging assay. The chemical profile of the extracts was determined using gas chromatography-mass spectrometry (GC-MS) analysis, and the antiproliferative activity was tested using MCF-7 (breast cancer) and MCF- 10A (normal breast) cell lines and the Sulphoromide B assay.
Results: The percentage of scavenging ability was 12.354 ± 0.003% and 13.151 ± 0.009% for ADDCM and ODDCM, respectively, with r2 values of 0.969 and 0.968. The total content of DPPH scavenging radicals in the ADDCM and ODDCM extracts significantly correlated with their trolox relaxation effects (r2 >0.9, p<0.05). GC-MS identified 89 major phytochemical compounds present in both extracts. The antiproliferative effects of both extracts on MCF-7 cells were dose dependent. No antiproliferative effect was detected for MCF-10A cells.
Conclusion: Both extracts exhibited free radical scavenging activity, but the ADDCM extract contained a greater amount of chemical compounds. Additionally, the DCM extracts showed selective antiproliferative activity against MCF-7 but not MCF-10A.
Clinacanthus nutans; Pre-extraction; SRB assay; DPPH scavenging assay; Soxhlet extraction
Scientific research has led to the development of many drugs derived from medicinal herbs, as numerous plants have been shown to contain phytochemical compounds that are beneficial to human health [1]. Plants traditionally have been used to maintain health, and modern research has safety tested many of them, showing that they have no adverse or toxic effects on human health. Currently, basic research on plants continues to add to databases that are used for bioinformatics studies at the cell and molecular level with the aim of developing new and effective drugs to treat human illnesses.
One of the plants of interest for cancer research is Clinacanthus nutans (CN) (family Acanthaceae), which is a medicinal herb that is called ‘bone grass’ in China [2] and Sabah snake grass in Malaysia and Indonesia [3]. Its popular name in Malaysia is ‘belalai gajah’. CN inhabits areas with sandy and peat soils. It is characterized by full of green leaves, and the entire plant can grow to 1–2 meters long. Various phytochemical compounds have been detected in CN, including beta-sitosterol, 132-hydroxy-(132-S)-phaeophytin a, (3β)- Lup-20(29)-en-3-ol, clinacoside A, clinacoside B, isomollupentin 7-O-β-glucopyranoside, vitexin, isovitexin, orientin, isoorientin, shaftoside, clinamide A, clinamide B, clinamide C, 2-cisentadamide A, and apigenin 6,8-C-α-L-pyranararabinoside [4,5].
Today there is great demand for medicinal herbs to prevent and treat health problems, especially cancer, and studies of CN are increasing because of its potential as a medicinal herbal treatment for cancer. In Southeast Asia, CN is used in complementary medicine for treating cancer patients due to the presence of compounds such as quercitine, catechin, and luteolin, which are thought to induce cancer cell apoptosis and inhibit cancer growth [6]. Quercitine and luteolin are compounds in the flavonoids group that are used as anticancer agents. In a review of antioxidant activity of CN, Ariful Alam [7] reported that the presence of flavonoids and flavone resulted in higher radical scavenging activity. Other compounds in CN include triterpenes, flavone glycoside, glucoside, and thioglycoside [8]. The potential pharmacological effects of CN are antivirus, antibacterial, antioxidant, antiproliferative, antiinflammatory and anti-herpes [9].
CN is used traditionally in Thailand to treat herpes simplex virus [10], diabetes, blood pressure, and as an antilytic and diuretic [9,11]. Juiced leave are used as a preventative and a treatment for cancer, and in Malaysia and Singapore the juice often is prepared by blending fresh leaves with green apple to reduce the bitter taste and grassy smell of the leaves [12]. Additionally, leaves are applied to problem areas to treat snake bites, rashes, and inflammation [6].
Discoveries of therapeutic benefits of medicinal plants start with the pre-extraction and extraction of the sample. The pre-extraction process aims to avoid damage to the biomolecules in the plants. Fresh or dried plant parts can be used for the extraction, during which the separation of active compounds follows standard procedures with selective solvents [13]. The extraction method provides alkaloids, glycosides, phenolics, terpenoids and flavonoids, which are active plant metabolites. In medicinal plant studies, the pre-extraction and extraction processes are very important. The efficiency and phytochemical constituents obtained by the final extraction are affected by the pre-extraction procedure, which involves sample preparation (e.g. grinding or drying). Each extraction procedure is unique, and no specific universal extraction method is used for all plants. Thus, optimization experiments needed to develop an effective method for a given plant. The objective of this study was to test the effects of oven versus air drying (i.e., variations of a preextraction parameter) on the antioxidant activity, chemical profile, and antiproliferative activity of the Dichloromethane (DCM) extract of CN leaves obtained using the same soxhlet extraction method.
Plant material
CN leaves were collected in May 2014 at HERBagus Farm Pongsu Seribu, 13200 Kepala Batas, Seberang Perai Utara, Pulau Pinang, Malaysia. Dr. Rahmad Zakaria identified the plant, and a specimen was deposited at the Herbarium Unit, School of Biology, Universiti Sains Malaysia under voucher number 11536.
Preparation of plant extract
Two types of drying process were tested in this study: air drying and oven drying. Once this pre-extraction process was completed, the same DCM extraction method was used to produce the CN extracts. CN leaves were weighed and washed with distilled water. Air dried leaves were left to dry under free air until they were completely dry, whereas oven dried leaves were dried in an oven at 40°C until they were completely dry.
For each sample type, dried leaves were grounded to powder using a blender. The samples then were extracted using DCM for 24 h at a rate of three cycles per hour in a Soxhlet extraction apparatus. Each extract type then were filtrated and concentrated used a rotary evaporator until complete dried separately. The stock solution was 500 μg/mL (dissolved in dimethyl sulfoxide) and it was filtered through a 0.45 μm syringe filter. All extracts were stored at 4°C until further analysis.
Antioxidant activity
The capacity of the antioxidants in the extracts was assessed by measuring their free radical scavenging effect of the 2,2-diphenyl-1- picrylhydrazyl (DPPH) radical with trolox as reference compound. This assay was carried out according to the method described in the Sigma protocol (1898-66-4) (D9132) (C18H12N5O6) (Mw: 394.33). The DPPH microplate assay was conducted in 96-well plates with successive sample dilutions consisting of 0.02, 0.04, 0.07, 0.13, 0.25, 0.5, and 1 mg/mL. Each extract concentration (50 μL) was mixed with 200 μL of DPPH solution (0.2 mM DPPH in 100 mL of methanol). The mixture was incubated in the dark for 30 min at 30°C. The absorbance was measured at 517 nm against a blank of methanol without the DPPH solution using microplate reader (Biotek, Model ELx808, Agilent Technologies, Wilmington, DE, USA). Trolox was used as standard references and control. Results were expressed as percentage of inhibition of the DPPH radical, which was calculated as follows:
% scavenging of DPPH = (Abs control–Abs sample/Abs control) × 100
Where Abs control is the absorbance of the DPPH solution without extracts. The capacity of the extracts to reduce DPPH was obtained from the standard curve, and the results are expressed as % inhibition against extract concentration.
Gas chromatography-mass spectrometry (GC-MS) analysis
The GC-MS analysis was performed following the method described by Mohammad Hafiz Abdul Rahim [14]. The samples were analyzed using an Agilent 7890A Gas Chromatograph (Agilent Technologies, Wilmington, DE, USA). For analysis, a 1 mg aliquot of a given extract was weighed into a GC vial and mixed with 1 mL of methanol. A sample volume of 10 μL then was injected in splitless mode into the GC-MS system, which was connected to a MS/MS triple quad detector. The GC column used for the analysis was an HP-5MS, with an inner column and film thickness diameter of 0.25 mm × 30 m × 0.25 μm. The initial oven temperature was set to 100°C for 2 min, and it was increased to a target temperature of 280°C for 17 min at a rate of 10°C/min. Helium was used as the carrier gas at a rate of 1 mL/min. Each sample was analyzed in triplicate. The spectra for each of the chromatogram peaks were compared with the National Institute of Standards and Technology (NIST) database library and the Retention Time (RT) index of common primary and secondary metabolites.
In vitro antiproliferative activity assay
Human breast epithelial adenocarcinoma cells (MCF-7) and normal breast cells (MCF- 10A) were used throughout this study. These cell lines were purchased from American Type Culture Collection (Manassas, VA, USA) with code numbers ATCC®HTB- 22TM and ATCC® CRL-10317TM, respectively. Both cell lines originated from humans, are adherent cells, and are from breast epithelial mammary glands. MCF-7 breast cancer cells overexpress estrogen receptor, whereas MCF-10A cells are nontumorigenic and nonmetastatic epithelial breast cells. These cells were maintained in complete growth medium in a 5% CO2 atmosphere at 37°C in a humidified incubator and passaged every 2-3 days.
MCF-7 cells were maintained in complete growth medium formulated with ATCC-formulated Eagle’s Minimum Essential Medium (EMEM, Catalog No. 30-2003). The two supplements added to create complete growth medium were 0.01 mg/mL of human recombinant insuline and fetal bovine serum with a final concentration of 10%.
MCF-10 cells were maintained in complete growth medium with base medium (MEBM), Lonza with kit MEGM
(CC-3150, Lonza) which contains bovine pituitary extract, human recombinant epidermal growth factor, insuline, hydrocortisone, gentamycin-amphotericin B mix, and 100 ng/mL of cholera toxin.
Cells were seeding in 96 wells plate in 100 μL completed growth media at plating densities. That cells than incubated at 37°C, 5% CO2, 95% air and 100% relative humidity for 24 h prior to addition of ADDCM and ODDCM extracts. The extracts were treated in 72 hours. The SRB assay was performed essentially. The cells were fixed with 50% cold trichloroacetic acid and incubated for 30 min at room temperature. The plates were washed five times with distilled water and dried, and then the cells were stained with 100 μL of 0.4% SRB in 1% acetic acid for 30 min at room temperature. The cells then rinsed four times again with 1% acetic acid. After the plate had dried, 100 μL of 10 mM Tris buffer were added. The plates were shaken for 5 min, and absorbance at a wavelength of 540 nm was read using a microplate reader (BMG LABTECH, Germany). Cell survival was measured as the percentage absorbance compared to the untreated control.
Statistical analysis
All the data were analyzed used SPSS for window v24. All data presented as the mean ± Standard Deviation (SD) for triplicate determination. Significant of data was determined by t-test for antioxidant activity and one way ANOVA post hock turkey for antiproliferative effect. P values ≤ 0.05 was assumed as statistically significance value.
Antioxidant activity of the CN extracts
Figure 1 shows the percentage of DPPH radical scavenging of the ADDCM and ODDCM CN extracts, respectively.
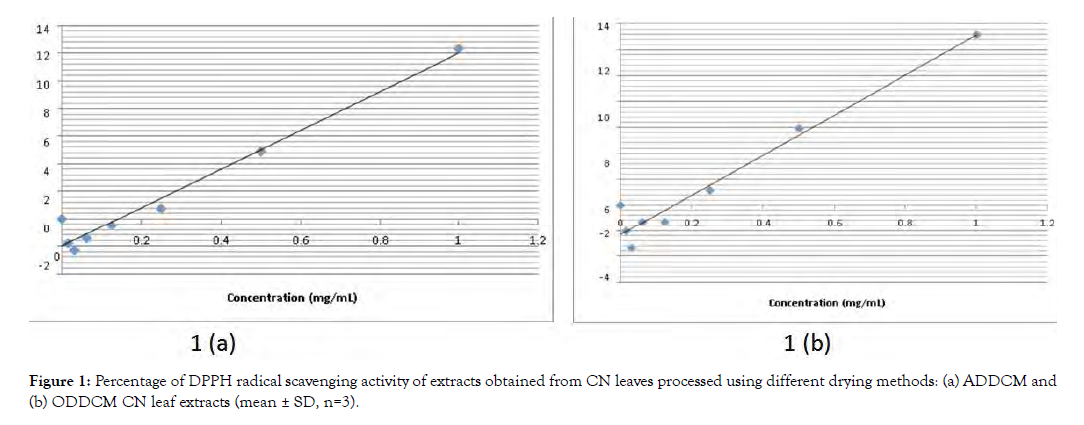
Figure 2: Percentage of DPPH radical scavenging activity of extracts obtained from CN leaves processed using different drying methods: (a) ADDCM and (b) ODDCM CN leaf extracts (mean ± SD, n=3).
Chemical composition of the CN extracts
GC-MS identified 89 major phytochemical compounds present in both extracts. Figure 2 shows the chromatogram of volatile compounds in the ADDCM and ODDCM CN extracts. Additionally, Tables 1 and 2 list the volatile organic compounds present in the ADDCM and ODDCM CN extracts, respectively. Table 3 shows the similarity of volatile compounds present in the two types of extract.
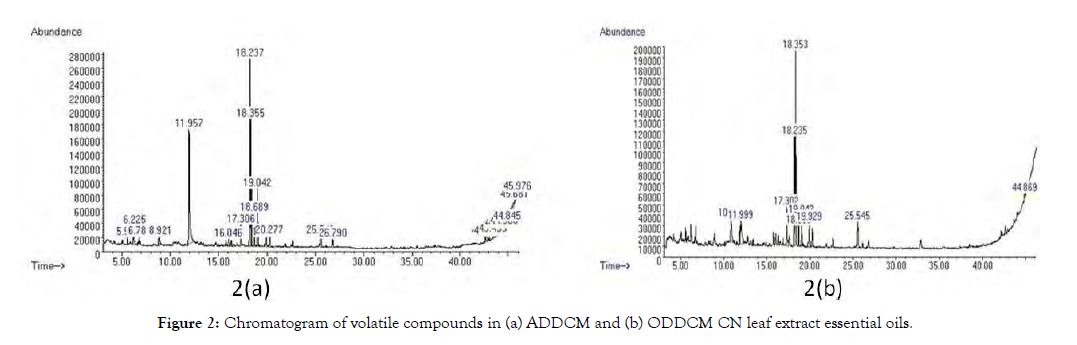
Figure 2: Chromatogram of volatile compounds in (a) ADDCM and (b) ODDCM CN leaf extract essential oils.
| Volatile compounds | RT (min) | Area (%) |
|---|---|---|
| 1-Hexanol,2-ethyl- | 5.584 | 0.96 |
| S-Methyl methanethiosulphonate | 6.225 | 3.58 |
| Nonanal | 6.781 | 0.71 |
| Piperazine, 2-methyl- | 6.781 | 0.71 |
| Quinoline-5,8-dione-6-ol, 7-[[(4yclohexybutyl)amino]methyl]- | 6.781 | 0.71 |
| O-Ethyl methylphosphinic acid) | 8.839 | 1.10 |
| Oxirane, (butoxymethyl)- | 8.839 | 1.10 |
| Benzaldehyde,2-nitro-, diaminomet hylidenhydrazone | 8.839 | 1.10 |
| alpha.-(Aminomethylene)glutaconic | 8.924 | 1.54 |
| Cyclopropane,ethylmethylene- | 8.924 | 1.54 |
| 1,3-Pentadiene, (Z)- | 8.924 | 1.54 |
| o-Toluidine | 11.96 | 22.59 |
| Cycloheptane, 4-methylene-1-methyl-2-(2-methyl-1-propen-1-yl)-1-viny l- | 11.96 | 22.59 |
| Pyrazine, ethyl- | 11.96 | 22.59 |
| 2-Oxo-3-methyl-cis-perhydro-1,3-benzoxazine | 16.043 | 1.15 |
| 6H-Pyrazolo[1,2-a][1,2,4,5]tetrazine,hexahydro-2,3-dimethyl- | 16.043 | 1.15 |
| 1-Dodecanamine | 16.043 | 1.15 |
| Hepta-2,4-dienoic acid, methyl ester | 17.308 | 2.96 |
| Boroxin, ethyldimethyl- | 17.308 | 2.96 |
| Benzene, 1,2-dimethoxy-4-(1-propenyl)- | 17.308 | 2.96 |
| Bicyclo[3.1.1]heptane, 2,6,6-trimethyl(1.alpha.,2.beta.,5.alpha.) | 18.236 | 22.84 |
| Bicyclo[3.1.1]heptane, 2,6,6-trimethyl- | 18.236 | 22.84 |
| Bicyclo[3.1.1]heptane, 2,6,6-trimethyl-,[1R-(1.alpha.,2.beta.,5.alpha.)]- | 18.236 | 22.84 |
| 2-Pentadecanone, 6,10,14-trimethyl | 18.354 | 15.12 |
| Cyclohexane, 1-methyl-4-(1-methylethenyl)-, trans- | 18.691 | 4.21 |
| Cyclohexanol, 1-ethynyl- | 18.691 | 4.21 |
| Bicyclo[3.1.1]heptane, 2,6,6-trimethyl-, (1.alpha.,2.beta.,5.alpha.) | 18.691 | 4.21 |
| Cyclohexanol, 1-ethynyl- | 19.046 | 6.97 |
| Bicyclo[4.1.0]heptane, 3-methyl- | 19.046 | 6.97 |
Table 1: Volatile organic compounds present in the ADDCM CN extract.
| Volatile compounds | RT (min) | Area (%) |
|---|---|---|
| Cyclohexane, 1,3-dichloro-, cis- | 10.847 | 7.44 |
| Sulfurous acid, diethyl ester | 10.847 | 7.44 |
| Oxirane, (ethoxymethyl)- | 10.847 | 7.44 |
| Benzo[1,2,5]thiadiazole-4-sulfonic acid (pyridin-3-ylmethyl)-amide | 11.994 | 10.28 |
| N-m-Tolyl-succinamic acid | 11.994 | 10.28 |
| Pyridine, 2,3-dimethyl- | 11.994 | 10.28 |
| 1,2,5-Oxadiazol-3-amine, 4-[5-(2-t hienyl)-1,2,4-oxadiazol-3-yl]- | 17.308 | 8.27 |
| 1-Methyl-3-n-propyl-2-pyrazolin-5-one | 17.308 | 8.27 |
| 2(4H)-Benzofuranone, 5,6,7,7a-tetr ahydro-4,4,7a-trimethyl-, (R) | 17.308 | 8.27 |
| Bicyclo[3.1.1]heptane, 2,6,6-trimethyl-, (1.alpha.,2.beta.,5.alpha.) | 18.236 | 19.29 |
| Bicyclo[3.1.1]heptane, 2,6,6-trimethyl- | 18.236 | 19.29 |
| Bicyclo[3.1.1]heptane,2,6,6-trimethyl-,[1R-(1.alpha.,2.beta.,5.alpha.)]- | 18.236 | 19.29 |
| 2-Pentadecanone, 6,10,14-trimethyl | 18.354 | 32.65 |
| N(Epsilon)-methyl-l-lysine | 18.691 | 3.46 |
| 9-Octadecene, 1,1-dimethoxy-, (Z)- | 18.691 | 3.46 |
| 1-Heptadecanamine | 18.691 | 3.46 |
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 19.046 | 5.04 |
| Cyclohexanol, 3-(3,3-dimethylbutyl)- | 19.046 | 5.04 |
| 1,4-Eicosadiene | 19.046 | 5.04 |
| Hexadecanoic acid, methyl ester | 19.923 | 6.32 |
| Pentadecanoic acid, 14-methyl-, methyl ester | 19.923 | 6.32 |
| Phytol | 25.541 | 6.99 |
| Tetrasiloxane, decamethyl-b) | 44.873 | 0.26 |
| Cyclotrisiloxane, hexamethyl- | 44.873 | 0.26 |
| Methyltris(trimethylsiloxy)silan | 44.873 | 0.26 |
Table 2: Volatile organic compounds present in the ODDCM CN extract.
| S. No. | Volatile Compounds | Group Compounds |
|---|---|---|
| 1 | Bicyclo[3.1.1]heptane,2,6,6trimethyl(1.alpha.,2.beta.,5.alpha.), | Terpenes |
| 2 | Bicyclo[3.1.1]heptane,2,6,6-trimethyl, | Terpenes |
| 3 | Bicyclo[3.1.1]heptane, 2,6,6-trimethyl-,[1R-(1.alpha.,2.beta.,5.alpha.)]-, | Terpenes |
| 4 | 2-Pentadecanone, 6,10,14-trimethyl, | Acetone |
| 5 | Hexadecanoic acid, methyl ester, | Ester |
| 6 | Pentadecanoic acid, 14-methyl-, methyl ester, | Ester |
| 7 | Phytol | Terpenoid |
| 8 | Cyclotrisiloxane, hexamethyl- | Alkane |
| 9 | Tetrasiloxane, decamethyl | Alkane |
Table 3: Similar volatile compounds present in both ADDCM and ODDCM CN extracts.
Antiproliferative effect of the CN extracts
Figure 3 shows the antiproliferative effects of the CN extracts on MCF-7 cells, and Figure 4 shows their antiproliferative effects on MCF-10A cells. The antiproliferative effects of both extracts on MCF-7 cells were dose dependent, but no antiproliferative effect was detected for MCF-10A cells.
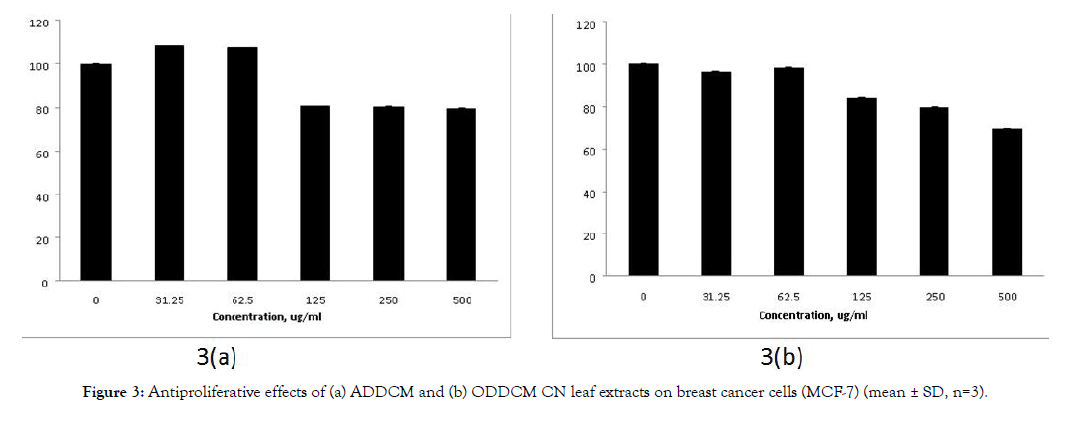
Figure 3: Antiproliferative effects of (a) ADDCM and (b) ODDCM CN leaf extracts on breast cancer cells (MCF-7) (mean ± SD, n=3).
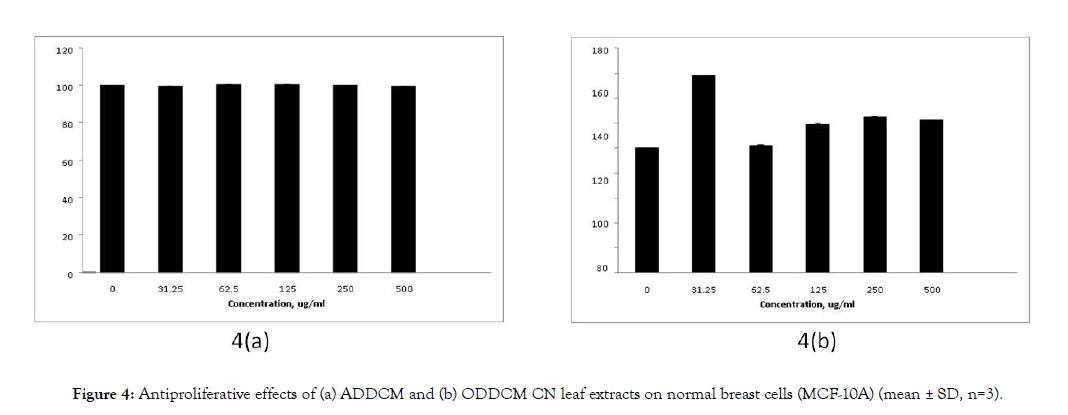
Figure 4: Antiproliferative effects of (a) ADDCM and (b) ODDCM CN leaf extracts on normal breast cells (MCF-10A) (mean ± SD, n=3).
The DPPH radical scavenging assay is commonly used to screen for antioxidant components of plant extracts [15]. In this study, the radical scavenging ability of both the ADDCM and ODDCM samples occurred in a concentration-dependent manner (Figure 1). Although the percentage of radical scavenging activity in the ODDCM samples (13.151%) was higher than that in the ADDCM (12.354%) samples, values for both were in the same range, and the percentage of scavenging ranged from 0.0153 to 1 mg/mL. The redox properties in radical scavenging act as free radical scavengers, hydrogen donors, metal chelators, singlet oxygen quenchers, and reducing agents [16]. Velayutham and Karthi [17] previously reported that CN has free radical scavenging activity as well as antiplasmodial, antiarthritic, antidiabetic, antimicrobial, anti-inflammatory, antifertility, anticancer, and nephron protective activities. Antioxidants in many plant extracts have been shown to have human health benefits [18], improve quality of life, and provide protection against human chronic diseases, including cancer, inflammation, and cardiovascular and neurodegenerative diseases [15].
GC-MS is an effective platform for secondary metabolite profiling in plant and nonplant species [19], and it is also a useful way to extract polar solvents and produce volatile oils with bioactive compounds [20]. In this study, many volatile compounds were identified by GC- MS analysis based on similarity matching with the NIST library. In total, 89 total compounds were found in the ADDCM and ODDCM extracts, but the quantitative and qualitative results were higher for the ADDCM extract than for the ODDCM extract (Figure 2). In the ADDCM extract, 64 compounds were identified (Table 1), whereas only 25 compounds were detected in the ODDCM extract (Table 2). The three major compounds in the ADDCM samples (constituting 18.236% of the area) were Bicyclo[3.1.1]heptane, 2,6,6-trimethyl(1. alpha., 2. beta., 5.alpha.), Bicyclo[3.1.1]heptane, 2,6,6-trimethyl-, and Bicyclo[3.1.1] heptane, 2,6,6-trimethyl-,[1R-(1.alpha.,2.beta.,5. alpha.)]-. In the ODDCM samples, the major component was 2-Pentadecanone, 6,10,14-trimethyl, and it constituted a higher percentage area (19.29%). This results shows due to the presence of major compounds with a high percentage area and the presence of other compounds to the synergy among the compounds [21]. Nine compounds were present in both extract types, and they were terpenoids, acetone, esters, and alkanes (Table 3). The ester is under group of terpenoids. The terpenoid group compounds were Bicyclo[3.1.1]heptane, 2,6,6-trimethyl(1.alpha.,2.beta.,5.alpha.), Bicyclo[3.1.1]heptane,2,6,6-trimethyl, Bicyclo[3.1.1]heptane, 2,6,6-trimethyl-,[1R-(1.alpha.,2.beta.,5.alpha.)]-, and phytol while ester group compounds were hexadecanoic acid, methyl ester and pentadecanoic acid, 14-methyl-, methyl ester. The acetone group member was 2-Pentadecanone, 6,10,14-trimethyl, and the alkanes were cyclotrisiloxane, hexamethyl- and tetrasiloxane, decamethyl. The activity of the extract may be due to the synergic effect of ester and acetone compounds in both extracts.
The SRB assay was used to study the antiproliferative effect of the extracts against breast cancer cells (MCF-7) and normal breast cells (MCF-10A). The extract concentrations tested were 0, 31.25, 62.5,125, 250 and 500 μg/mL. The ADDCM and ODDCM extracts exhibited antiproliferative activity on MCf-7 cells, and the inhibition occurred in a dose dependent manner (Figure 3). However, no inhibition effects were observed for the ADDCM and ODDCM extracts on MCF-10A cells (Figure 4). This indicated that both extract samples were safe for normal cells, possibly due to their antioxidant activity and the presence of volatile compounds.
In this study, many of the terpenoids present in both sample extracts showed the potential of the extract for use in cancer research. Terpenes are a group of terpenoids that generally are bound to sugar moieties by a glycoside linkage, and they have weak central nervous system activity and strong molluscicidal and fungitoxic activities. They also may have chemopreventative properties [22,23]. However, it has proved difficult to identify synergisms or antagonisms that may occur in oils, and reports that have used terpenes as standards are lacking [22].
Ester compounds are in the terpenoids group, and several ester compounds (hexadecanoic acid, methyl ester, and pentadecanoic acid, 14-methyl-, methyl ester) were found in the extracts. In a study of Euphoria kansui that characterized and isolated methyl esters and derivatives, n-hexadecanoic acid (such as palmitic acid), octadecanoic acid (such as oleic acid), and octadecanoic acid (such as stearic acid) were the major fatty acids in the ester group, which previously had been reported to have antioxidant activities and anticancer [24]. However, Henry et al. [25] found that n-hexadecanoic acid had 68% antioxidant activity, 9-octadecanoic acid had only moderate activity, and n-octadecanoic acid had poor activity. In other studies, n-hexadecanoic acid was reported to have ~60% antioxidant activity [26], cytotoxic activity [27], and antiinflammatory, antispasmodic, and antiviral activities [28].
In the current study, 2-pentadecanone, 6, 10, 14-trimethyl, which is a member of the acetone group, was detected in the extracts. This active compound has an antiproliferative effect, as reported by Nikoline Fri Tanih and Roland Ndip Ndip [29], who found that the acetone extract of stem bark of Sclerocarya birrea showed good antiproliferative effects on three cell lines (MCF-7, HeLa, and HT- 29). Alkane group compounds (cyclotrisiloxane, hexamethyl- and tetrasiloxane, decamethyl) present in both extract types also have potential anticancer effects.
Plants have secondary metabolites called isoterpenoids, also known as terpenoids, and many of them exhibit cytotoxicity against a variety of cancers and tumours. Terpenoids have no side effects on normal cells and therefore are safe, and they have potential antimicrobial, antifungal, antiparasitic, antiviral, antiallergic, antispasmodic, antihyperglycemic, anti-inflammatory, and immunomodulatory properties [30]. The phytochemical anticancer agents currently being explored in clinical trials in China and India are mainly terpenoids and include about 40,000 individual compounds [28]. In the current study, both extract types (ADDCM and ODDCM) contained high amounts of terpenoids and exhibited antiproliferative effects on MCF-7 cells but no effects on MCF- 10A cells. This information helps improve the understanding of mechanisms associated with terpenoid compounds and their biological role in the prevention and treatment of cancer [31].
Both of the extracts evaluated in this study were extracted via DCM Soxhlet extraction. The DCM extracts were polar compounds, and polarity of an extract is an important characteristic because the polarity of the solvent plays a major role in extraction of specific compounds. Moreover, the isolation of phyto compounds is confirmed by polarity-based extraction [32]. Some studies have shown that DCM extraction can extract flavonoids, aldehydes, nitrogen compounds, fatty acids, and fatty acid esters. However, in this study the major compounds in both extracts were terpenoids, including the subdivision containing esters (other terpenoid subdivions are alcohols, aldehydes, ketones, ethers, phenols, and epoxides) [33].
The search for plants with anticancer properties is based on analysis of active compounds and their derivatives present in plant extracts [29]. The pre-extraction and extraction processes used to obtain compounds from natural products are an integral part of the discovery process. Samples available for analysis include fruits, flowers, leaves, bark, and roots, and fresh or dried material can be used for extraction. The pre-extraction preparation plant material influences the preservation of phytochemicals obtained in the final extraction [1]. In this study, samples were either air dried or oven dried during the pre-extraction process, but they were extracted using the same method. However, both samples affected DPPH activity and antiproliferative effects on breast cancer cells. DPPH radical scavenging in another way is caused by the mechanism of reaction widely used in accessing free radical scavenging [34] and the contributions to overall activity by creating synergistic effects [35]. The presence of secondary metabolites and antioxidant activity due to chemical structure and redox properties of compounds in an extract also can result in synergistic effects [34]. The antioxidant activities of polar and non-polar compounds in plant-derived essential oils occur because of the presence of active compounds and constituents as well as the synergy among them [21,35].
This study had several limitations that must be considered when interpreting the results. For example, during the air drying process samples were exposed to ambient temperature, thus samples may have been subjected to contamination and unstable temperature conditions [1]; however, heat-labile compounds were preserved because high temperature heating was not used. Another disadvantage of the air drying method is that samples take a long time to dry compared to microwave drying and freeze-drying. Preextraction processing using oven drying uses thermal energy and removes moisture from the samples, and it is considered to be the easiest way to rapidly process samples and preserve phytochemicals. However, oven drying may affect the phytochemicals. For example, studies of Orthosiphon stamineus reported no effect of drying method on antioxidant activity, but the sinensetin and rosmarinic acid contents were affected by sunlight and oven drying temperature [1,36]. Finally, Soxhlet extraction, also known as hot continuous extraction, was used in this experiment. The disadvantages of this extraction method are exposure to hazardous and flammable liquid organic solvents with potential toxic emissions. Thus, this system is not environmentally friendly, and advanced extraction techniques can require costly reagents [37]. Additionally, the procedure involves many factors, such as solvent sample ratio, temperature, and agitation that must be optimzed [38]. Nevertheless, Soxhlet extraction is widely used in industry because it is efficient, has good reproducibility, and requires less extract manipulation compared to other extraction methods [39,40].
Results of this study demonstrated that air dried and oven dried samples of C. nutans leaves subsequently extracted via DCM Soxhlet extraction exhibited free radical scavenging activity and contained bioactive compounds with potential anticancer properties. Moreover, both samples extracts showed selective antiproliferative activity against breast cancer cells but not normal breast cells. The compounds isolated from the C. nutans leaf extracts may prove to be natural antioxidants that can be used in the food, pharmaceutical, and supplement industries.
This work was supported and funded by a FRGS grant (203/ CIPPT/6711340) from the Ministry of Higher Education, Government of Malaysia and a USM Bridging Grant (304/ CIPPT/6316239) to Hasni Arsad.
Citation: Md. Toha Z, Haron NH, Md. Kamal NNS, Arsad H (2020) Antioxidant, Antiproliferative Activities and Chemical Profile of Clinacanthus nutans Leaf Extracts Processed Using Two Different Pre-Extraction Drying Methods. Med Aromat Plants (Los Angeles) 9: 361. doi: 10.35248/2167-0412.20.9.361.
Received: 19-Aug-2020 Accepted: 19-Sep-2020 Published: 25-Sep-2020 , DOI: 10.35248/2167-0412.20.9.361
Copyright: © 2020 Md. Toha Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.