Journal of Plant Biochemistry & Physiology
Open Access
ISSN: 2329-9029
ISSN: 2329-9029
Research Article - (2019)Volume 7, Issue 4
Double haploid (DH) plants Production is a valuable tool in plant breeding programs. Since conventional breeding takes long time for the development of newly improved cultivars, this technique reduces the time needed for this purpose. Practically, application of (DH) technology in potato breeding through androgenesis in Santana cultivar is possible by considering number of factors that influence androgenesis. Culture medium has to be considered in respect of constituents to fulfill the needs of culture target where N6 medium significantly showed better results (14.61%) if compared to MS medium (8.78%) anthers produced embryos. Auxin/cytokinin represented in this study by 2,4-D/BA showed better results in induction of embryogenesis when combined together at (2.0 mg/l 2,4-D and 0.5 mg/l BA) which resulted in (35.67%) anthers produced embryos rather than each one of them alone. Enhanced results were obtained after floral buds pretreatment with thermal chock with three different degrees (4°C, 25°C, 30°C) for two different exposure time (48 h and 72 h). N6 medium showed the highest percentage of anthers produced embryos (44%) when supplemented with 2 mg/l 2,4-D+0.5 mg/l BA, while pretreatment with 4°C for 72 h favored the embryos production (16.67%) followed by (14.00%) and (12.00%) at 25°C and 4°C for 48 h respectively with no significant difference among them. Pretreatment with 32°C for (48 h and 72 h) resulted in (4.67%) and (5.33%) respectively differed significantly form the low temperature. Silver nitrate has shown to be potent at 2 mg/l in inhibiting ethylene, increasing embryogenesis (60.0 %) and reducing the non-responsive anthers (6.67 %).
Androgenesis; Callus induction; Embryogenesis; Potato (Solanum tuberosum)
Potato (Solanum tuberosum L.) is a major tuber food crop in the world belong to the family Solanaceae. As a globally important crop in regard of production, it ranks the fourth most important food crop in the world after wheat, rice and maize [1]. Globally Potato production is about 388 million tons planted on nearly 45 million feddans (feddan=0.42 ha) of cultivated land. In Egypt, potato also ranks the fourth in production, production is trending a head annually where cultivated area is about 400.000 feddan producing about 4,325,478 tons [1]. Potato has an economic importance in Egypt and any disturbance in its production affects severely its local and export trade.
Common cultivated potato species have a base chromosome number of n=12 and may be diploid (2n=2x=24), triploid (2n=3x=36), tetraploid (2n=4x=48) or pentaploid (2n=5x=60) [2]. Potato has an extremely large secondary gene pool consisting of related wild species, the majority of wild species are diploid [3]. About 64% of wild Solanum species are diploid (2x=24), with most of the remaining species tetraploid (4x=48) or hexaploid (6x=72) [4]. This genetic diversity has proven to be valuable in breeding programs in addition to disease resistance, environmental tolerance, and other agronomic traits such as processing qualities of interests [5].
Among powerful tools used in plant breeding and provided by biotechnology, tissue culture, in specific, haploid and doubled haploid technology which helping to improve plants selection. If implemented in breeding programs, doubled haploids will reduce the time needed for conventional breeding programs [6].
Haploids are plants with gametophytic chromosome number (n) which after duplication retain the (2n) chromosome number again. This biotechnological tool led to use of doubled haploids technology to unlock genetic variation. In root crops, this technology has been recently integrated with breeding programs that will open new opportunities for improving of selection methods, maximizing selection gains and accelerate cultivar development with single step of homozygous lines in compare to conventional breeding methods which requires several generations of selfing [7].
Anther culture is based on the in vitro development of microspores in anthers into sporophytic-phase plants. This technique attracted attention as the most preferred technique to produce haploids due to its high frequency of producing haploid plants [8]. It is also a potent and efficient way to produce homozygous true-breeding progeny lines in plant breeding programs [9]. Several researchers considered anther culture an easy and one step program for regenerating haploid plant [10]. Androgenesis delivers new inbred lines carrying desirable traits which can be introgressed into responsive clones. Hereby, anther culture is a potent key to develop germplasm of potato by conversion to a diploid crop [11].
Microspore which undergo androgenesis (haploid induction and subsequent regeneration) develops in two pathways, either to develop into haploid callus or into haploid embryo, then both pathways regenerates into a haploid plants [12].
Several factors affecting androgenisis such as genotype of the donor plants, physiological condition of donor plants, developmental stage of microspores, pre-treatment of flower buds, composition of the culture medium and physical factors during tissue culture [13].
Advantages of generating dihaploids form tetraploids cultivars in potato, either by anther culture or by using specific pollinators such as Solanum phureja, is overcoming obstacles of development inbred lines, plus controlling the cross incompatibility caused by the differences in species ploidy levels and endosperm balance numbers [14]. This will enlarge the potato gene pool and introgression of new valuable traits. In addition, acceleration of homozygous lines development and selection for biotic and abiotic stresses resistant plants after homozygous lines crossing [15].
The objective of this study is aimed to induce androgenesis in Solanum tuberosum, cultivar Santana, through four steps: (1) Examination the effect of salt media (MS or N6) types on induction of embryogenesis from anthers, (2) examination of growth regulators (GRs) (2,4-D and BA) effect. (3) Examination of pretreatments with temperature at different degrees (4°C, 25°C, 32°C) and time of exposure (48 or 72 h) and (4) examination of silver nitrate (AgNO3) effect on induction of embryogenesis from anthers.
Experiment (1): Effect of media types (MS and N6) supplemented with different concentrations of BA and 2,4-D on percentage of anther produced embryos of Santana cv
The main effect of each growth regulator and media type on embryogenesis. Two weeks post culture, most anthers showed different color and were noticed to be swollen and formed either a round mass shape (Figure 2A) or in longitudinal shape (Figure 2B). After one month, most of the anthers produced callus which appeared either compact and green, or friable and white.
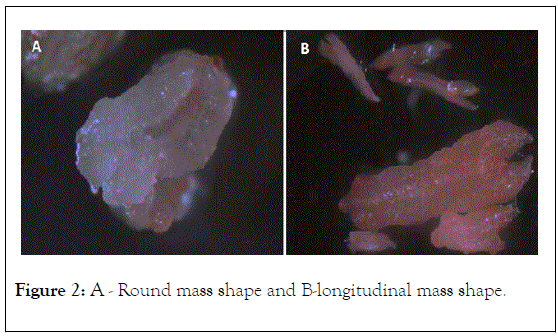
Figure 2: A - Round mass shape and B-longitudinal mass shape.
Table 1 Illustrate the effect of media types and its combination with 2,4-D and BA on the percentage of anthers produced embryos. The highest percentage of anthers produced embryos was recorded by N6 medium (14.61 %) as compared to MS medium (8.78 %). Maybe this could be attributed to the different composition of each medium in concern of nitrogen source ratio between nitrate (NO3-) and ammonium (NH4+) which is in accordance with Grimes and Hodges [19] explanation about the high response to embryogenesis in rice when cultured on N6 medium in compare to MS medium, because of the difference of NO3- to NH4+ ratio in MS and N6 (2:1) and (4:1) respectively. This ratio showed best response to auxin 2,4-D due to NO3 - radicle at 0.5 mg/l 2,4-D.
| Media Types (A) | 2,4-D mg/I (B) | Mean % of anthers produced embryos | Means of (A × B) |
Means of (A) | ||
|---|---|---|---|---|---|---|
| BA mg/I (C) | ||||||
| 0 | 0.5 | 1 | ||||
| MS | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 8.78 |
| 0.5 | 1.33 | 3.33 | 2.67 | 2.44 | ||
| 1 | 8.67 | 16.00 | 12.67 | 12.44 | ||
| 2 | 12.00 | 27.33 | 21.33 | 20.22 | ||
| N6 | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 14.61 |
| 0.5 | 0.67 | 6.00 | 2.67 | 3.11 | ||
| 1 | 16.67 | 24.67 | 23.33 | 21.56 | ||
| 2 | 22.67 | 44.00 | 34.67 | 33.78 | ||
| Means of ( c ) | 7.75 | 15.17 | 12.17 | Means of ( B ) | ||
| Means of (B × C) | 0 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.5 | 1 | 4.67 | 2.67 | 2.78 | ||
| 1 | 12.67 | 20.33 | 18.00 | 17.00 | ||
| 2 | 17.33 | 35.67 | 28.00 | 27.00 | ||
| (A × C ) | MS | 5.50 | 11.67 | 9.17 | ||
| N6 | 10.00 | 18.67 | 15.17 | |||
LSD at 5 %(A=2.18)(B=3.08)(C=2.67)(A × B=4.36)(A × C=3.77)(B × C=5. 34)(A × B × C=7.55)
Table 1: Effect of media (MS and N6) and different concentrations of BA and 2,4-D on percentage of anther produced embryos of Santana cv. after four and half months in vitro.
Appropriate concentration of growth regulators in the medium is an important effective factor in callus formation in anther culture, especially 2,4-D which plays an important role the process of dedifferentiation of cells [20].
The main effect of 2,4-D (disregard of BA concentration) showed that the highest (27.0 %) and the lowest (2.78 %) percentage of anthers produced embryos were recorded at levels of 2.0 mg/l and 0.5 mg/l 2,4-D and BA respectively, this could be attributed to 2,4-D role in the dedifferentiation of plant cells converting it to embryogenic cells. This is in accordance with Rihova and Tupy [21] who reported that 2,4-D free medium failed to form embryos in potato anther culture. In the same context the embryos formation was increased gradually in response to the gradual increase of 2,4-D concentration and this is in agreement with results of Kaur et al. [22] who studied the effect of 2,4-D on somatic embryogenesis in potato. However, Nowaczyk et al. [23] reported that 2,4-D is used as supplement in medium as an essential factor for embryos development in anther cultures of pepper.
The main effect of BA (disregard of 2,4-D concentration) showed that highest (15.17 %) and the lowest (12.17 %) percentage of anthers produced embryos were recorded at levels of 0.5 mg/l and 1.0 mg/l as shown in table 1. Embryos formation increased significantly from (7.75 %) and (15.17 %) anthers produced embryos at levels of 0.0 to 0.5 mg/l BA. This is in accordance with Cheng et al. [24] who reported that BA is capable of inducing embryogenesis in microspores culture of pepper at levels of 0.2-0.4 mg/l BA and that changing its levels affect embryogenesis significantly On the other hand, at level of 1.0 mg/l this capability decreased significantly when compared to 0.5 mg/l. This results are in harmony with Aboshama [25] who reported that BA increased embryos formation in volkamer lemon which was diminished with increase of BA concentration.
In the same table, the effect of the interaction between the two growth regulators 2,4-D and BA on the anther produced embryos showed that highest (35.67 %) and the lowest (1.00 %) percentage of anthers produced embryos resulted from (2 mg/l 2,4-D/0.5 mg/l BA) and (0.5 2,4-D/0.0 mg/l BA) respectively,
Interaction between the two GRs revealed that combination of 2.0 mg/l 2,4-D and 0.5 mg/l BA differed significantly form all other combinations including the control. embryos formation at tested combinations were significantly high if compared to either 2,4-D or BA effect alone. This may be due to the effect of auxin and cytokinin on cells divisions and elongation plus the stress created by GRs in in medium. This is on the same line with Qin and Rotino [26] who reported that BA at 0.6 mg/l alone or BA in combination with 2.4-D promotes embryogenesis in sweet and hot pepper. In the same context Sharma et al. [27] reported the role of 2,4-D in somatic embryo induction of potato. However, the role of 2,4-D in induction of somatic embryos can be explained according Bogre et al. [28] who reported that 2,4-D promote cell division and affect endogenous levels of natural auxins in alfalfa. On the other hand, Ardebili et al. [29] reported the importance of 2,4-D as a new stress factor enhances embryogenesis in microspores of rapeseed.
Direct embryo formation could be noticed without callus formation. Well-formed dicot embryos showed normal developmental patterns into globular, heart, torpedo and cotyledonary stages. Conversion of embryos into vigorous doubled haploid plantlets could be achieved with colchicine treatment (Figure 3).

Figure 3: Doubled haploid plantlets.
Effect of interaction between GRs and media types on embryogenesis: Percentage of anther produced embryos was determined after four and half months as shown in Table 2. Interaction of media types and GRs showed that N6 medium recorded the highest percentage of anthers formed embryos (44 %) and the lowest was (0.67 %) were acheived at (2.0 mg/l 2,4-D and 0.5 mg/l BA) and (0.5 mg/l 2,4-D and 0.0 mg/l BA) respectively. While in MS medium the highest (27.33 %) and the lowest (1.33 %) percentage of anthers formed embryos were recorded when supplemented with the same concentrations of both GRs. N6 medium differed significantly from MS medium when both were supplemented with the same GRs combinations. this result is in agreement with Aboshama [25] findings, where N6 basal medium proved to be more conducive to the induction of volkamer lemon embryos in anther culture than MS when both were supplemented with the same combination of BA and 2,4-D.
| Media | 2,4-D mg/1 | BA mg/1 | Mean % of anthers Produced callus | Mean % of anther produced embryos | Mean % of non-responsive anthers |
|---|---|---|---|---|---|
| MS | 0 | 0 | 0.00 | 0.00 | 100.00 |
| 0.5 | 5.33 | 0.00 | 94.67 | ||
| 1 | 7.33 | 0.00 | 92.67 | ||
| 0.5 | 0 | 26.00 | 1.33 | 72.67 | |
| 0.5 | 27.33 | 3.33 | 69.33 | ||
| 1 | 32.00 | 2.67 | 65.33 | ||
| 1 | 0 | 47.33 | 8.67 | 44.00 | |
| 0.5 | 53.33 | 16.00 | 30.67 | ||
| 1 | 51.33 | 12.67 | 36.00 | ||
| 2 | 0 | 57.33 | 12.00 | 30.67 | |
| 0.5 | 60.00 | 27.33 | 12.67 | ||
| 1 | 42.67 | 21.33 | 36.00 | ||
| N6 | 0 | 0 | 0.00 | 0.00 | 100.00 |
| 0.5 | 20.00 | 0.00 | 98.00 | ||
| 1 | 8.67 | 0.00 | 91.33 | ||
| 0.5 | 0 | 9.33 | 0.67 | 90.00 | |
| 0.5 | 20.00 | 6.00 | 74.00 | ||
| 1 | 46.00 | 2.67 | 51.33 | ||
| 1 | 0 | 45.33 | 16.67 | 38.00 | |
| 0.5 | 37.33 | 24.67 | 38.00 | ||
| 1 | 42.00 | 23.33 | 34.67 | ||
| 2 | 0 | 56.00 | 22.67 | 21.33 | |
| 0.5 | 35.33 | 44.00 | 20.67 | ||
| 1 | 36.00 | 34.67 | 29.33 | ||
| LSD | 7.9 | 7.55 | 12.55 |
Table 2: Effect of different concentrations of 2,4-D and BA, media (MS and N6) on Percentages of anthers produced callus, embryos and that did not response in Santana cv.
After 30 days, callus induction rates were determined as shown in table 2. MS medium recorded highest (60.0 %) and lowest (5.33 %) percentage of anthers formed callus at levels of (2.0 mg/l 2,4-D and 0.5 mg/l BA) and (0.0 mg/l 2,4-D and 0.5 mg/l BA) respectively. While N6 recorded highest (56.0 %) and lowest (2.0 %) percentage of anthers formed callus at levels of (2.0 mg/l 2,4-D and 0.0 mg/l BA) and (0.0 mg/l 2,4-D and 0.5 mg/l BA) respectively. The highest percentage of anthers formed callus in both media didn’t show any significant difference from each other.
On the other hand, when both media MS and N6 are compared at the same GRs levels (2.0 mg/l 2,4-D and 0.5 mg/l BA) which resulted in (60.0 %) and (35.33 %) anthers formed callus respectively, MS medium was superior and differed significantly form N6 medium. These results were in accordance with Bregitzer [30] who reported that MS medium has a known ability and can be used in callus formation in barley.
MS medium superiority to N6 medium considering anther produced callus if compared at the same combination levels of 2,4-D and BA is in accordance with Singh et al. [31] and Kawochar et al. [32] who induced callus successfully on MS medium in different cultivars of potato. In contrast Zhao et al. [33] reported that N6 medium was effective in callus induction with coneflower anthers in compare to MS medium. However, results indicated that MS and N6 medium were the best for callus induction which is in conformity with Ullah et al. [34] who demonstrated that best response to callus induction in rice was found on both media MS and N6 at (2 mg/l 2,4-D) and (0 or 0.5 mg/l BA). On the same line Hoque et al. [35] reported the importance of nutrient media type as one of the key factor of callus induction in water chestnut.
Experiment (2): Effect of thermal pretreatment with (4°, 25° and 32°C) and exposure time (48 h and 72 h) on anthers produced embryos
This experiment shed the light on the effect of pretreatment with heat shock and chilling treatments of anthers on the embryo formations in Santana cv. Anthers pretreated with low temperature for both durations were more responsive to embryogenesis than anthers treated with high temperature for both durations which resulted in brown anthers.
Anthers were pretreated with 3 levels of temperatures degrees (4°, 25° and 32°C) represents thermal treatments with heat shock or chilling treatments for two different durations (48 h and 72 h).
Table 3 illustrates the main effect of pretreatment with different temperature levels and durations of exposure on anthers produced embryos. Applying this pretreatment comes in accordance with Murovec and Bohanec [13] who demonstrated that thermal shock is the most widely used method as effective pretreatment factor to induce pollen embryogenesis development.
| Pretretments (A) | Mean Percentage of anthers produced embryos | Means of (A) | |
|---|---|---|---|
Time(B) |
|||
| 48 h | 72 h | ||
| 4°C | 12.00 | 16.67 | 14.33 |
| 25°C | 14.00 | 10.67 | 12.33 |
| 32°C | 4.67 | 5.33 | 5.00 |
| Means of (B) | 10.22 | 10.89 | |
LSD at 5 % A=1.686 B=3.42 Ab=5.92
Table 3: Effect of pretreatment and their time of exposure on percentage of anthers produced embryos of Santana cv after 4 and half months in vitro.
The main effect of 4°C (disregarding the exposure time) resulted in (14.33 %) anthers produced embryos with significant increase in compare to (12.33 %) and (5.0 %) at 25° and 32°C respectively. While the main effect of exposure time (disregarding the temperature) either 48 h or 72 h showed no significant difference from each other and resulted in (10.22 %) and (10.98 %) anthers derived embryos respectively.
In the same table the interaction between temperature and time of exposure showed that highest anthers produced embryos was (16.67 %) when anthers were exposed to 4°C for 72 h, while the lowest was (5.33 %) at 32°C for 72 h. The former pretreatment result is followed by treatment result of 4°C for 48 h (12.0 %) and 25°C for 48 h which resulted in (14.0 %) with no significant difference among them. This may be due to the positive response to the interaction between solanaceous spp. and pretreatment with low temperature. This is in combatable with Tiainen and Veilleux [36,37] who illustrated the importance of pretreatment of solanaceous species with low temperature (4-6°C) for 2-4 days in the dark to enhance embryogenesis stimulation. In contrast Heidari-Zefreh et al. [38] found that cold pretreatment at 4°C in sweet pepper, decreased anther derived embryos sharply for the same plant.
Pretreatment with 32°C for 48 h or 72 h showed the lowest percentage of anthers produced embryos (4.67 %) and (5.33 %) respectively. This is in accordance with Chlyah et al. [39] who reported that pretreatment with high temperature was unfavorable for tomato and led to browned anthers. In contrast, Vural et al. [40] reported that eggplant as a solanaceous species did not respond to cold pretreatment which was not suitable, while 35°C for 8 days under dark conditions gave better results and enhanced embryos formation. In the same context Qi et al. [41] reported that anthers of french marigold pretreated with 31°C for 1 day turned brown and eventually died.
Prereatments used in this study comes in accordance with Jacquard et al. [42] who reported that anther pretreatment is important step for barley anthers, as stress is necessary to switch the gametophytic program of the microspores to the sporophytic developmental pathway. This reorientation give rise to haploid embryoids. However, Tai and Xiong [43] have reported that in potato anther culture a low temperature (4-5°C for 72 h) as well as high temperature (35°C for 12 h) can be used as a pretreatment. Cold and heat chock stress treatment of anthers induces microspores embryogenesis. Microspores switches (reprograming) development from gametophytic to the embryogenic pathway as mentioned by Rodriguez-Serrano et al. [44] Positive response to androgenesis was described by Wang et al. [45] by preventing microspores death which increase viable microspores by suppressing the pathway of microspores development into maturity.
Some mechanisms are involved in pretreatment effect on androgenesis such as: 1) blocking accumulation of starch in vegetative cytoplasm of binucleate pollen, which block pollen maturity pathway as reviewed by Maraschin et al. [46] 2) Stress factors induce cytoskeletal and nuclear rearrangements in microspores and slowing down degradation of anther tissues which protect microspores from toxic substances released from the decaying anthers, thus prolong the survival of microspores Shariatpanahi et al. [47] 3) Cold and heat chock treatment also increases the levels of intracellular ABA, as reported by Vishwakarma et al. [48] that plant cell produce ABA (important in normal growth of somatic embryos) in response to a range of stresses such as cold temperature.
Experiment (3): Effect of silver nitrate (AgNO3) (0.0, 1.0, 1.5 and 2.0 mg /l) on embryogenesis
In this experiment it was noticed that plantlets are healthier without showing wilting or vetrification, in addition embryos formation increased as well as leaf area.
Data in table 4 elucidate the effect of silver nitrate (AgNO3) addition with different levels (0.0, 1.0, 1.5 and 2.0 mg /l) on percentage of anthers produced embryos. Generally, anthers produced embryos was increasing ascendingly in response to addition of (AgNO3), where highest response was (60.0 %) at treatment of 2 mg/l which differed significantly if compared to all other treatment levels. This may be attributed to the ethylene inhibition characteristic of silver ion in an readily translocated form (AgNO3), which allowed plantlets to grow well. This is in consistence with Sandra and Maira [49] who reported that plantlets in potato culture treated with 2 mg/l (AgNO3) showed no epinasty or hyperhidricity symptoms caused by ethylene and resulted in highest leaf area when compared to non-treated culture.
| AgNO3 | Mean % of anthers produced callus | Mean % of anther produced embryos | Mean % of non responsive anthers |
|---|---|---|---|
| 0 | 30.67 | 44.00 | 25.33 |
| 1 | 34.67 | 53.33 | 12.00 |
| 1.5 | 36.00 | 50.00 | 14.00 |
| 2 | 33.33 | 60.00 | 6.67 |
| LSD | 4.42 | 5.84 | 7.63 |
Table 4: Effect of silver nitrate (AgNO3) concentrations on the embryo yields in Santana cv.
On the same line this could be explained in the light of previous work done by Abdolvand et al. [50] who indicated that AgNO3 have a positive effect on embryogenesis of date palm due to its strong inhibitory effect on ethylene by reducing its negative impact and confers in vitro growth and development.
Embryos were not only formed at cultures treated with different levels of (AgNO3), but also formed in the control treatment in the absence (AgNO3). Results of this study suggests that (AgNO3) may not be essential factor for induction but for increasing embryos yield from anther culture which by turn will decrease the non-responsive anthers of Santana cv. Similar results were described by Kabir et al. [51] who reported that addition of (AgNO3) (the ethylene inhibitor) showed progressive increase in embryo yields in anther culture of Brassica rapa ssp significantly if compared to control which had less embryos yield.
Silver nitrate is known to be a potent inhibitor of ethylene action in plants as indicated by Kumar et al. [52] who emphasised that (AgNO3) is very potent inhibitor of ethylene action and is used widely in plant tissue culture.
Several literatures mentioned that silver ions masks the activity of ethylene gas and behaved as a strong inhibitor resulting in improved and microspore embryogenesis Kabir et al. [51]. This is positively reflected on the progressive reduction of nonresponsive anthers in the present study where the percentage of non- responsive anthers decreased in response to the increasing of (AgNO3) concentrations.
Plant material
Healthy and vigorously growing flower buds of potato cultivar Santana was harvested at florescence stage and were used as plant materials. Santana cultivar is imported from Stet Holland Co., Netherland, and planted in Wadi El Natroun farm in Egypt.
Pollen developmental stage
Flower buds were collected from healthy plants from Wadi El Natroun farm in Egypt. Buds were in different sizes (Figure 1A) which may be synchronized indirectly with different stages of pollen development. To test the stage of pollen development, anthers (one anther per bud) were subjected to cytological examination by squashing and staining with 1% acetocarmine in 45% acetic acid and observed under the microscope at 100x magnification in order to identify the uninucleate or tetrad stage of pollen development [16]. Based on acetocarmine test, tetrad stage was found to occur when flower buds are of 4-6 mm long (Figure 1B). For subsequent experiments, only flower buds of 4-6 mm size were used.
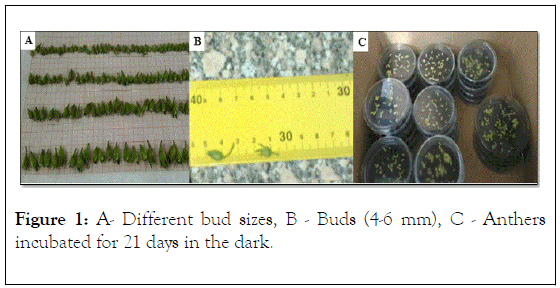
Figure 1: A- Different bud sizes, B - Buds (4-6 mm), C - Anthers incubated for 21 days in the dark.
Experiments (1): Effect of salt media (MS, N6) and growth regulators (2,4-D, BA) on induction of androgenesis
In order to examine the effect of (MS and N6) media and growth regulators (2,4-D and BA) on induction of haploid embryos. Two types of salt media, MS and N6 [17,18]. were used to determine the best medium for formerly mentioned purpose. Both media were supplemented with growth regulators (GRs) namely benzyladenine (BA) at (0.0, 0.5 and 1.0 mg/l) at all possible combinations with 2,4-dichlorophenoxyacetic acid (2,4- D) at (0.0, 0.5, 1.0 and 2.0 mg/l). Media were solidified with 7.0 g/l agar and supplemented with 60 g/l sucrose, 200 mg/l ascorbic acid, 500 mg/l casein hydrolysate, 200 mg/l Lglutamine, 1.0 mg/l thidiazuron (TDZ) and 3.0 g/l activated charcoal. The pH of media was adjusted to 5.8 prior to the addition of agar. The media were autoclaved at 121°C and 1.5 kg/cm2 air pressure for 20 min. The media were distributed in petri dishes (10 cm - diameter) containing 15 ml of solid medium. 30 anthers were placed in each petri dish.
Anther culture
The collected floral buds were washed with sterile distilled water for 5 min to remove the dust, then the unopened buds were surface sterilized by immersion for 30 sec. in 70% (v/v) ethyl alcohol followed by immersion in 20% commercial Chlorox (5.25% NaOCl) for 15 min with a drop of Tween 20. Eventually the flower buds were washed 4 times in sterile distilled water to remove all traces of the disinfectant. After removing petals aseptically by a small forceps, anthers were dissected carefully and filaments were removed while keeping anthers intact without injuries and then cultured in Petri dishes. Re-culture took place every 5 weeks for 4 times. At the end of culture period anthers produced callus or embryos were calculated in percentage, also non-developed anthers either to callus or to embryos (nonresponsive) were calculated.
Culture conditions
The Petri dishes with anthers were sealed with Parafilm, incubated initially at 25 ± 2°C for 21 days in the dark (Figure 1C), followed by incubation under a 16/8-h photoperiod with light supplied by cool-white fluorescent lamps.
Experiment (2): Effect of pretreatments temperature (4°C, 25°C, 32°C) and exposure time (48 or 72 h) on percentage of anthers produced embryos
Pretreatments of floral buds with temperature at different degrees (4°C, 25°C, 32°C) and time of exposure (48 or 72 h) were examined to investigate the best combination of both and its effect on increasing callogenesis and embryogenesis. In this experiment buds were harvested and stored (pretreated) at 4°C, 25°C and 32°C in darkness for 48 and 72 h, after that, anthers were cultured on the best induction medium (N6 medium supplemented with 2.0 mg /l 2,4-D+0.5 mg/l BA) resulted as described in the previous experiment. All petri dishes were transferred and maintained at dark conditions on 25 ± 2°C for further 21 days, followed by incubation under a 16/8-h photoperiod with light supplied by cool-white fluorescent lamps. Temperature degrees and exposure time were evaluated as percentage of anthers produced embryos after four and half months.
Experiment (3): Effect of silver nitrate (AgNO3) (0.0, 1.0, 1.5 and 2.0 mg/l) on embryogenesis
Based on the two former experiments, the best induction medium (N6 medium supplemented with 2.0 mg/l 2,4-D+0.5 mg/l BA) from the first experiment was used to examine the effect of (AgNO3) on induction of embryogenesis from anthers which were submitted to the best pretreatment (4°C for 72 h) form the second experiment. Different concentration of (AgNO3) were added to the culture media (0.0, 1.0, 1.5 and 2.0 mg /l). experiment procedures were carried out (as described above) immediately post culturing, petri dishes were incubated at 25 ± 2°C. Data were collected four and half months later and calculated as percentage of anthers producing callus or embryos plus the anthers which didn’t response as well.
Embryo germination
Embryos were transferred to germination medium contains MS salt plus vitamins, 30 g/l sucrose, 1.0 mg/l gibberelic acid (GA3) and 7 g/l agar.
Colchicine treatment
As soon as embryos were germinated and reached 4-5 mm in size, they were transferred to culture tubes contained the regeneration medium of 1/4 MS supplemented with 200 mg/l colchicine in order to obtain doubled haploid plantlets by doubling the chromosome numbers of dihaploid embryos. Embryos were incubated for 4 weeks in the light at 25 ± 2°C under 16/8-h photoperiod.
Plant recovery
About 5 cm in length Plantlets which developed good roots were transferred to the greenhouse in order to transplant them for the acclimatization phase in pots containing mixture of equal volume of peatmoss and vermiculite.
Statistical data analysis
Experiments were set up in completely randomized block design with five replicates; each replicate consisted of one Petri dish containing 30 anthers, Statistical analysis performed. Data were analyzed using the SPSS® program. Analysis of variance was determined using the general linear model procedures and means were separated with LSD test.
N6 nutrient medium has provided better result in regard to embryos formation and the overall androgenesis process of anther culture in Santana cultivar. N6 medium supplemented with plant growth regulators 2,4-D and BA, enhanced embryogenesis and results were better by than the N6 alone. Pretreatment of anthers as proved by many researchers is essential process although it differs in respect to the temperature and exposure time. This study showed that pretreatment with 4°C for 72 h significantly resulted in the highest percentage of anthers produced embryos in Santana cultivar. Silver nitrate is a potent inhibitor to ethylene enabling callus and embryos formation by suppressing the senescence of plant tissues, where 2 mg/l has shown to be effective in this respect. Previous studied factors showed that improving haploid production in Santana cultivar is possible by different combination of embryogenesis controlled factors, further studies is recommended in another processing cultivars.
Citation: AboShama HM, Atwa MM (2019) Anther Culture in Potato (Solanum tuberosum L.) in vitro. J Plant Biochem Physiol. 7:244. doi: 10.35248/2329-9029.19.7.244
Received: 05-Dec-2019 Accepted: 19-Dec-2019 Published: 26-Dec-2019
Copyright: © 2019 AboShama HM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.