Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Review Article - (2018) Volume 9, Issue 1
The assessment of water quality is one of the important tools for sustainable development and provides decisive information for water management. In identifying water availability for irrigation, knowledge is required on both the quantity and quality; nevertheless, quality need has often been neglected especially in developing countries. Quality should generalize how substantially a water supply fulfils the needs of the planned user and must be assessed based on its suitability for the proposed use. The quality of groundwater is determined by various physicochemical properties and chemical indices. Chemical properties of groundwater are controlled by natural geochemical processes and anthropogenic activities. Therefore, to monitor the quality of groundwater for irrigation purposes, in depth understanding of hydro geochemistry of the water is required since different ions in water have contrasting impacts on soil. The water having high sodium adsorption ratio (SAR) when used for irrigation heighten exchangeable sodium percentage (ESP) in the soil thereby adversely affecting the soil structure leading to decrease in infiltration, hydraulic conductivity, enhance surface runoff and erosion. Moreover, the poor-quality water causes impairment in crop growth as high salt concentration in irrigation water results in osmotic stress and ion toxicity in plants.
<Keywords: Ground water; Irrigation; Hydro geochemistry; Osmotic stress
Water required for irrigation of cropped land is being degraded in terms of quantity and quality due to ever-increasing demand for the use of water in the contemporary societies. Furthermore, the crop productivity is linked both with the quality of soil and the quality of the water available for irrigation. In general, evaluation of irrigation water quality should focus on salt content, sodium concentration, the occurrence of nutrients and trace elements, alkalinity, acidity, and hardness of the water. Every year throughout the globe salinity leads to the loss of fertile soils [1,2]. Groundwater is the leading source of water supply for domestic, industrial and agricultural sectors of many countries situated in arid zones [3]. In identifying water availability for irrigation, knowledge is required on both the quantity and quality; nevertheless, quality need has often been neglected especially in developing countries. Quality should generalize how substantially a water supply fulfils the needs of the planned user and must be assessed based on its suitability for the proposed use [4].
The quality of water focuses on its suitableness for use. Maximum yields can be obtained if the water quality is good under proper soil and management conditions. The salt problem in soils occurs when the water applied for irrigation contains more soluble salts which get accumulated in the root zone as the plants absorb water thereby reduces yield. Severe water scarcity is arising in various parts of the world, particularly in arid and semi-arid regions. The overdependence on groundwater to meet ever-increasing demands of domestic, agriculture, and industry sectors has resulted in overexploitation of groundwater resources in these areas. The suitability of water for irrigation is determined by the concentrations of some elements that contribute to the specific conductance of groundwater. Particularly, higher concentration of sodium causes dispersion and swelling of soil which is inevitably unfavorable thereby leading to surface crusting decrease infiltration rates at the surface and reduce the hydraulic conductivity of the soil [5].
The groundwater contamination due to salinization is major issue which can be triggered by various processes, viz., intrusion of seawater, pollution by agrochemicals, geogenic contamination and salinization induced by irrigation [6]. The ratio of sodium ions to calcium and magnesium ions can be used to prognosticate the degree to which irrigation water tends to enter the cation-exchange reactions in soil [7]. This ratio, called the sodium-adsorption ratio (SAR), is used to determine the sodium hazard for irrigation waters. Since, sodium hazard increases as SAR increases; therefore, the suitability of water for irrigation decreases. The effect of irrigation water on soil infiltration rates is dependent upon the interaction between the flocculating effects of specific conductance and the dispersion effects of sodium. Soils can tolerate irrigation waters with large SAR values if the specific conductance values are also large [5]. Besides water quality, various factors like type of soil, type of crop, crop pattern, precipitation etc. play a significant role in determining the suitability of water for irrigation [8]. Keeping in view the facts it is imperative to review the quality of groundwater for irrigation.
It is well documented that the mineral composition of water has profound effect on soil structure and plant growth. Hence, the classification system to assess the quality of water for irrigation can be ascertained as explained below.
Hydrogen ion activity or pH
The parameter pH is the negative logarithm of hydrogen ion activity. The pH scale ranges from 0 to 14. If pH is less than 7 it is considered as acidic in nature, greater than 7 is alkaline and pH of 7 is treated as neutral. Therefore, pH is a measure of acidity or alkalinity of water. The principal use of pH is quick evaluation of the possibility of water being normal or abnormal. The normal range of irrigation water is from pH 6.5 to 8.4 [4]. The pH of water changes with the production of hydrogen or hydroxyl ion in different chemical reactions. With the redox potential, temperature and pressure, the pH ascertains the compounds dissolved and precipitated in groundwater regime. In ground water the redox potential depends on the number of cations and anions in solution. Hence, the chemical concentration of anion with respect to pH may be inkling to its solubility [9]. The pH of water furnishes critical information in many types of geochemical equilibrium or solubility calculations [10]. Acid water has the potency to be corrosive to plumbing and faucets especially when the pH is below 6 [11]. As the pH of the irrigation water increases above 8.2, the potential for sodium problems enhances [12]. The higher pH of groundwater may be due to considerable sodium, calcium, magnesium, carbonate and bicarbonate concentration as carbonates and bicarbonates are hydroxyl generating ions [13-15]. Peseyie and Rao assessed the quality of groundwater in Dimapur, Nagaland and reported that the pH values varied from 4.5 to 7.0 indicating acidic to neutral nature; moreover, 66.04% of the water samples were acidic, whereas 33.96% were neutral [16].
Electrical conductivity
The term "electrical conductivity" is synonymous with "specific electrical conductance." The standard unit for conductivity is mhocm−1 but is also represented by dSm−1. Electrical conductivity is usually used for indicating the total concentration of the ionised constituents of natural water. It is closely related to the sum of the cations (or anions) determined by chemical analysis and, therefore, correlates, well with the value for dissolved solids [17]. Salinity alters accessibility of water to crops. Osmotic pressure of the soil water is increased by the excess salts thereby reduces absorption of water by plant roots, which results in a physiological drought condition. Even though the soil appears to have enough moisture, the plants may wilt because the roots do not absorb sufficient water to compensate for the water loss by transpiration. For diagnosis and classification, specific conductance is used to express the total concentration of soluble salts (salinity hazard) in irrigation water [18]. Electrical conductivity has been universally accepted as a standard measure of water quality, but there is a great degree of variability in choosing the water classes on this basis. The electrical conductivity is an index of degree of mineralization. Electrical conductivity varies with concentration, degree of ionization of the constituents and temperature [9]
Sharma reported that 4.7, 6.8, and 4.4% water from of irrigation circle of Bhakra system in Kaithal district (Haryana) had EC values less than 2.0, between 2 to 4 and more than 4.0 dSm−1, respectively with SAR problems [19]. Nishanthiny et al. ascertained the water quality of selected wells in Jaffna, Sri Lanka and based on EC, 44% of the wells had medium salinity water, 47% of the wells had high salinity water and 9% of the wells had very high salinity water [1]. Ahamed et al. reported that EC varied from 982-5422 μS cm−1 during the premonsoon and 1,170-3,495 μS cm−1 in the post-monsoon season; in Alathur Block, Tamil Nadu; with most of the samples having EC greater than 250 μS cm−1 which resulted in moderate-to-low crop productivity in both the seasons and ascribed the difference in EC to wide variation in surface and subsurface environments [20,21]. It is reported that in Obuasi Municipality of Ghana EC varied from 187.3 to 538.60 μS cm−1 with an average value of 142.03 μS cm−1 and 112.42 to 407.28 μS cm−1 with an average value of 178.4 μS cm−1 for dry and rainy seasons, respectively and ascribed the higher average value of EC in the rainy season to the enrichment of salt due to evaporation effect followed by dilution through rainwater [22].
Total dissolved solids (TDS)
The mineral components dissolved in water comprise dissolved solids. TDS are determined by evaporating the filtered sample of water to dryness, thereafter, weighing the residue [17]. The concentration of dissolved solids in natural water is normally less than 500 mg/l, however, water with greater than 500 mg/l is unsuitable for drinking and many industrial uses. Therefore, the total concentration of dissolved minerals in water is a universal indication of the over-all suitability of water for various uses [23]. The high TDS concentration in groundwater may be due to the occurrence of bicarbonates, carbonates, sulphates, chlorides and calcium. The methods which remove TDS are reverse osmosis, electrodialysis, exchange and solar distillation process. High value of TDS influences the taste, hardness, and corrosive property of the water [24-27]. The total dissolved solids are of great significance in determining the quality of water as these indicate hardness of water [9]. Due to differences in the solubility of minerals TDS in water vary significantly in different geological regions [28,29].
Raju observed that the total dissolved solids (TDS), in Gunjanaeru River basin, Cuddapah District, Andhra Pradesh, estimated by residue on evaporation method varied from 87 to 1,126 mg l−1 with an average value of 444 mg l−1 and 95 to 1,009 mg l−1 with an average value of 375 mg l−1 for post- and pre-monsoon water samples, respectively [30]. The increase of TDS in post-monsoon season was on the higher side than the pre-monsoon season owing to mixing of surface pollutants during the infiltration and percolation of rainwater. Salts, which remain adhered in the interstice or pores in clay/shale as groundwater is evaporated or water table falls, get leached to groundwater during the rainy period. Hence, the post-monsoon waters had higher TDS levels compared to pre-monsoon season. Ackah et al. assessed the groundwater quality for drinking and agricultural purposes in the Ga East Municipality (Ghana) and reported that total dissolved solids varied from 110-1384 mg l−1 [31].
Sodium percentage (Na %)
This term is also referred to as soluble sodium percentage or percent sodium. It is a computed by the following equation [17],
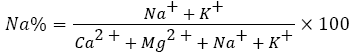
where the concentrations are in meq l−1. It is efficient in differentiating water because high value is the indication of soft water whereas a low value signifies hard water. Alkali (sodium) hazard is represented by sodium percentage, nevertheless, is not effective standard as sodium adsorption ratio [17] as percent sodium expresses percentage of sodium out of total cations in lieu of correlating sodium with calcium and magnesium only [18]. Nagaraju et al. reported that soluble sodium is important in categorizing irrigation water in terms of soil permeability i.e, ease with which a soil transmits water or air [32]. When irrigation water contains higher concentration of Na+, this ion tends to get adsorbed to the clay particles and displaces Ca2+ and Mg2+ ions. Exchangeable Na+ ions are attracted weakly to soil colloids, thus, spread out to form a comparatively thick swarm of hydrated ions held in very loose outer-sphere complexes around the colloids, contrary to Ca2+ which is strongly attracted to soil colloids by forming thin layer of hydrated ions. Therefore, highly sodium saturated colloids are held far apart that the forces of cohesion do not come into play to attract one colloid surface with another. Rather, the poorly balanced electronegativity of each colloidal surface repels other electronegative colloids and the soil becomes dispersed [33]. So, the exchange process of Na+ in water for Ca2+ and Mg2+ in soil decreases permeability consequently leads to soil with poor internal drainage. Hence, air and water movement are restrained during wet conditions and generally become hard when dried out [34,35]. Irrigation water having sodium percentage greater than 60 may lead to sodium accumulation and probably destruction of soil structure, infiltration and aeration [36,37]. The higher Na in the groundwater may be due to long residence time of water, dissolution of minerals from lithological composition and addition of chemical fertilizers with irrigation waters [38,39]. The importance of sodium concentration in classifying the water for irrigation use is imperative because sodium has profound effect on soil permeability and soil structure thereby results in little or no plant growth [40].
Raju while studying the quality of groundwater, in terms of percent sodium, in Cuddapah district of Andhra Pradesh revealed that out of 51 samples, 31 fall under excellent to good, 19 under good to permissible and one under permissible to doubtful, and out of 46 samples 40 fall under excellent to good, 5 under good to permissible and one under permissible to doubtful for post- and pre-monsoon seasons, respectively [30]. Pandian and Sankar classified groundwater on the basis of sodium percentage and found that out of 50 samples, 48 percent belonged to excellent to good and good to permissible types during post monsoon period but 44 percent in excellent to good and good to permissible types in pre-monsoon season whereas the remaining samples were classified as permissible to doubtful, doubtful to unsuitable and unsuitable types in both the seasons [41]. Nishanthiny et al. in Jaffna, Sri Lanka, reported that 3% of the wells had excellent irrigation water quality, 18% good, 44% permissible, 32% doubtful and 3% unsuitable irrigation water quality [1]. Bhat et al. reported that in Gohana, Haryana only 1.2% samples showed Na% within permissible limits, while as, 70.4% and 28.4% samples were doubtful and unsuitable for irrigation purposes, respectively [39]. Sudhakar and Narsimha (2013) ascertained the quality of groundwater in the Kushaiguda area Ranga Reddy District, Andhra Pradesh and reported that 37.5% of groundwater samples were in good to permissible category [42].
Sodium adsorption ratio (SAR) is the effective factor or parameter used for ascertaining the suitability of groundwater for irrigation purposes. Based on SAR values, irrigation water is classified into different classes which indicates that SAR value between 0-10, i.e., low sodium water poses almost no risk of exchangeable sodium, medium sodium water having SAR 10-18 can show considerable hazard, while on the contrary, high and very-high sodium water with SAR 18-26 and greater than 26, respectively are regarded as unfavourable as they can lead to detrimental levels of exchangeable sodium in soils [3,4]. Sodium Adsorption Ratio is calculated by the equation [43],
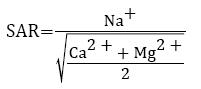
where all ionic concentrations are expresses in meq l−1. Ayers and Westcot reported that irrigation water having SAR values greater than 6-9 may induce permeability problem in swelling and shrinking types of soils [4]. In case of sensitive fruits, the tolerance limit of sodium adsorption ratio in irrigation water should not exceed 4, however, for general crops and forages a range of 8-18 is usually regarded as utile [44]. Yet, this indexing is applicable under soil conditions in a given area, so, demand regular field investigation. The sodium hazard of high carbonate waters is predicted by using SAR, particularly if the water contains no residual alkali [45]. Isaac et al. ascertained that the SAR of soil solution is increased with the increase in SAR of irrigation water which eventually increases the exchangeable sodium of the soil [46]. With elevated sodium adsorption ratio (SAR) values there is decrease in hydraulic conductivity and aggregate stability besides affecting clay dispersion, swelling of expandable clays, surface crusting and reduced tillage [47]. The toxicity of Na+ takes place with the accumulation of sodium in the tissues of plants thereby exceeding the tolerance limit of the crop. Moreover, excessive SAR levels can lead to soil crusting, poor seedling emergence, and poor aeration [48]. It is evinced that SAR can specify the degree to which irrigation water tend to enter cationexchange reactions in the soil [24].
Sudhakar and Narsimha ascertained the quality of groundwater in the Kushaiguda area Ranga Reddy District, Andhra Pradesh based on sodium adsorption ratio and reported that 69% of groundwater samples belong to S3C1, indicating high salinity and low alkali water [42].
Residual Sodium Carbonate (RSC)
For agricultural purposes, residual sodium carbonate (RSC) is usually used to ascertain the dangerous effect of carbonate and bicarbonate on the quality of water. Naseem et al. reported that pH, EC and SAR of the irrigation water are significantly influenced by RSC. The continuous usage of water having high RSC will cause burning of plant leaves and reduces the yield of crops [3,49]. Because of high concentration of bicarbonate in the water, the propensity for calcium and magnesium to precipitate increases as the water in the soil becomes more concentrated ensuing the decrease of permeability and finally leads to poor internal drainage of the soil [8,50,51]. Therefore, the relative content of sodium in the water is enhanced as sodium carbonate. The term RSC was suggested by Eaton [52] and is calculated by
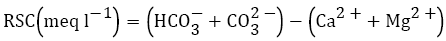
The land irrigated with water having high RSC assumes high pH; makes the soil infertile because of deposition of sodium carbonate as is recognized from the black colour of the soil [51]. Continuous irrigation with waters having RSC greater than 2.5 meq l−1 results in salt development which impedes the movement of air and water by clogging the soil pores [1,37]. The weakness of RSC is that it considers all bicarbonate in the water as though it would precipitate. However, the actual precipitation of bicarbonate depends upon the degree to which salts are concentrated in the root zone by evapotranspiration and if the solution exceeds saturation index of calcite. For instance, if the evaporation of water does not occur from the soil and the solution stays at equilibrium or unsaturated with respect to calcite, bicarbonate will pass through the soil. A negative RSC is the best condition because the total concentration of carbonate and bicarbonate is lower than the concentration of calcium and magnesium combined which implies that there is no residual carbonate to react with sodium to enhance the sodium hazard in the soil [53,54].
Based on RSC, Nishanthiny et al. in Jaffna, Sri Lanka evinced that 61% of the wells had good irrigation water quality, 15% doubtful and 24% had unsuitable irrigation water quality. Bhat et al. observed that 58% samples were of good quality and safe for irrigation, 4.9% were classified as permissible and 37% samples were unsuitable for irrigation based on RSC [1,39].
Infiltration
Infiltration is an important physical property which determines the water intake and surface run off the soil. The water with high salinity will increase infiltration whereas water having high SAR will decrease infiltration [55]. Rhoades and Oster and Schroer reported that slight infiltration problem occurred when ECW and SAR values were between 6-14 mScm−1 and >35 meq l−1, respectively [56,57]. Yeşilırmak evaluated the seasonal and spatial variations of water quality for irrigation in Büyük Menderes River, Turkey and reported that all waters had “no agricultural restriction” based on potential infiltration problem [58].
Lime Deposition Potential (LDP)
A special parameter, called as Lime Deposition Potential (LDP) is assessed to evaluate LDP from composition of cooling water [59]. Lime deposition potential (LDP) is lime deposition when calcium or magnesium carbonates (lime) precipitate out of the irrigation water, which leave behind white residues or deposits. Increasing temperatures, increasing pH, loss of carbon dioxide in the water and evaporation are the factors which are responsible for lime deposition. Any of these environmental effects can initiate deposition on the equipment, vegetation and fruit as water passes through the irrigation system [60]. The irrigation problems comprise plugging in microirrigation systems, precipitation of micronutrient fertilizers and SAR values [12]. Water with high LDP usually results in the formation of a spot of lime on fruits, which detract the value of crop [61].
Chloride
Owing to its high solubility in water chlorine exists as chloride ion and is the predominant natural form of chlorine [62]. Chloride salts are more harmful than sulphate. Since, when both these ions are present in high concentrations merely half of the sulphate leads to salinity because roughly half of the sulphates get precipitated as CaSO4 while the remaining half exists in soluble form as Na-MgSO4 in the soil [63]. Chloride is considered as the most common toxic ion in irrigation water. Since, chloride is not adsorbed by the soil colloids; therefore, it travels easily with soil water, is absorbed by the crop, moves into the transpiration stream, and accumulates in the leaves. If the chloride concentration in the leaves exceeds the tolerance of the crop, injury symptoms develop, such as leaf burn or drying of leaf tissue [4]. In all-natural waters chlorides occur in widely varying concentrations, the content of which by and large increases as the mineral content increases [29,64]. The concentration of chloride in natural waters is somewhat low except the water is brackish or saline [29,65]. In groundwater the origin of chloride may be from diverse sources such as weathering, leaching of sedimentary rocks and soils, intrusion of saltwater, windblown salt in precipitation, domestic and industrial waste discharges, municipal effluents, etc [66,67]. In groundwater the concentration of chloride ion upto 70 mg l−1 is considered safe and causes severe problem in the crops at concentration >350 mg l−1 [12]. The concentration of chloride in groundwater is best to study water-rock interaction [37].
Ackah et al. assessed the groundwater quality for drinking and agricultural purposes in the Ga East Municipality (Ghana) and reported that chloride varied from 28.41-813.8 mg l−1 [31]. Adhikary et al. while characterizing the groundwater quality for irrigation reported that chloride concentration varied from 0.70-153.55 me l−1 with a mean value of 11.54 me l−1 which is higher than the FAO standard of permissible limit for irrigation [29]. Bhat et al. while ascertaining the quality of water in Gohana, Haryana reported that 29.6% samples were highly suitable, 28.4% moderately suitable for irrigation [39]. However, 42% samples were unsuitable because of severe chloride hazard. Chloride in excess concentration can cause leaf burn if overhead irrigation is used and if taken up by the plant excessively. Yet, chloride is an essential micronutrient and the plant should be productive if chloride concentration is less than 70 ppm in irrigation water [60].
boron in soil solution may be detrimental to several crops. Boron is a micronutrient, therefore, necessary for growth of plants. However, slight excess of this element in the irrigation water or in soil solution can produce toxicity symptoms in certain crops [4]. Boron is an inorganic essential nutrient that is dissolved in water and exists as a mixture of the B10 (19.78%) and B11 (80.22%) isotopes [68,69]. The presence of naturally occurring boron in groundwater is primarily due to the leaching from rocks and soils containing borates and borosilicate’s. The possible sources of boron contamination in water resources are either natural (water rock interaction and seawater encroachment) or anthropogenic (sewage effluents and fertilizers [69,70]. Low saline groundwater contains low concentrations of boron in ppm [71] while high saline water might show high concentrations up to several tens of milligram per litre which 4.7 ppm for seawater is. The concentration of boron in groundwater ranges widely from <3 to >100 mg/l throughout the world [69]. McCarthy and Ellery proposed limits of boron concentration in irrigation water for semi-sensitive, semi-tolerant and tolerant crops as greater than 1.25, 2.5 and 3.75 ppm, respectively [72]. The most sensitive crops can tolerate no more than 0.5-1.0 ppm of boron concentration [14,73]. Subramani et al. reported that boron concentration in the groundwater of Chithar River Basin, Tamil Nadu, during July 2001 varied between 0-1.45 mg l−1 with mean value of 0.29 mg l−1. However, in July 2002, it varied from 0.02-1.03 mg l−1 with mean value of 0.33 mg l−1 [74].
Nitrate
During recent years, the pollution of groundwater by nitrates has been ascertained enormously across the globe [75]. The concentration of nitrate greater than 45 mg l‒1 causes a disease in humans called as methemoglobinemia or blue baby syndrome [76]. The possible origin of nitrate in agricultural areas include fertilizer, animal waste and mineralization of soil organic N (in plant residues, bacterial biomass and soil constituents). Due to intensive agriculture, large amounts of N fertilizers commonly urea, nitrate or ammonium compounds are applied which result in higher concentration of nitrate in the areas of intensive arable production [39,77]. The amount of nitrogen in groundwater is educed from the biosphere [35].
Sujata and Rajeswara investigated the water quality of south-eastern part of the Ranga Reddy district, Hyderabad, India and reported that the pollution regarding NO3- was mainly ascribed to the extensive usage of fertilizers and large-scale discharge of municipal wastes into the open drainage system of the area [78]. Ackah et al. assessed the groundwater quality for drinking and agricultural purposes in the Ga East Municipality (Ghana) and reported that nitrate varied from 1.9-4625 mg l−1 [31]. Bhat et al. reported that nitrate concentration in the groundwater samples of Gohana, Haryana varied from 0.59 to 55 mg l−1 with a mean value of 12.24 mg l−1 and concluded that 23.5% samples had no problem, 74.1% moderate and 2.5% had severe problem with respect to nitrate concentration [39]. In northern region of Jordan, Al-Tabbal and Al-Zboon (2012) observed that utilization of fertilizers is the main cause of nitrogen as high concentration of nitrate is an indicator of surface contamination source. Subramani et al. reported that the concentration of nitrate in groundwater samples, in Chithar River Basin, Tamil Nadu, during July 2001 varied from 1-52 mg l−1 with an average value of 21 mg l−1. In July 2002, it ranges from 0-56 mg l−1 with mean of 13.4 mg l−1 [74].
Magnesium Hazard (MH)
Broadly speaking, in groundwater alkaline earth metals are in state of equilibrium. Since, magnesium is an essential nutrient for plant growth and its deficiency causes yellowing and reduction in growth and yield of crops. The concentration of magnesium in water plays a pivotal role in deciding the quality of water for irrigation purposes, therefore, agricultural use [79]. Magnesium hazard of water for irrigation is calculated by the formula [80].
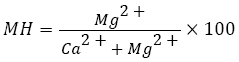
Magnesium hazard less than 50 is considered suitable for irrigation whereas greater than 50 is insidious and unsuitable for irrigation thus decreasing the yield of crops as soils become more alkaline. At the similar level of salinity and SAR, adsorption of sodium by soils and clay minerals is more at higher Mg: Ca ratios, since, the bonding energy of magnesium is lower than that of calcium, allowing more sodium adsorption and it occurs when the ratio surpasses 4 [81,82].
Pandian and Sankar observed that magnesium ratio of postmonsoon and that of the pre-monsoon groundwater samples varied from 21.55-92.81 and 35.02-91.44, respectively [41]. Magnesium ratios are found to be more than the permissible limit in all water samples locations, except southern and south-western parts of the Vaippar River Basin, Tamil Nadu in both seasons. The reaction and passage of surface water and subsurface water through limestone, kankar, and granitic rock formation is responsible for high Mg ratio in the study area. Ishaku et al. analysed groundwater quality in Jada and environs which revealed that magnesium ratio values varied from 34.3 to 82.5% with mean of 60.8% thus making it unsuitable for irrigation practice [83]. Al-Tabbal and Al-Zboon reported that in northern region of Jordan the values of MH varied from 65.9 and 2.65 with mean value of 39.97 and concluded that 84.5% of the samples have magnesium hazard values less than 50, therefore, can be classified as suitable for irrigation use [14].
Permeability Index (PI)
The long-term use of irrigation water had a profound effect on soil permeability as it is influenced by total dissolved salts, sodium content and bicarbonate content. Therefore, to integrate these three terms, Doneen empirically devised an equation referred to as ‘Permeability Index (PI)’ after carrying a series of investigations for which he used enormous number of irrigation water samples of variable ionic relationships and concentration [84].
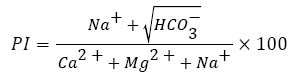
Permeability index is a crucial parameter for assessing the suitability of irrigation water. From the ecological viewpoint, in combination with subsurface structural features high permeability index would facilitate extensive contamination of groundwater [14]. In accordance with PI, water can be classified as Class I, II and III. Class I and II water are categorized as good for irrigation with 75% or more of maximum permeability. Class III water is unsuitable with 25% of maximum permeability.
Aghazadeh and Mogaddam reported that in Oshnavieh plain of Iran permeability index varied from 30% to 66% with an average value of 43% during May 2006 which entails that the groundwater of in the study area can be designated as class II (25-75%) thus suitable for irrigation purposes [85]. Ishaku et al. analysed groundwater quality in Jada and environs which revealed that permeability index values ranged from 24.1 to 254.3% with mean of 93% thereby making it unsuitable for irrigation practice [83]. Subramani et al. reported that permeability index in Chithar River Basin, Tamil Nadu varied from 25.8% to 86.3% during July 2001 and 11.6% to 86.9% during July 2002 [74].
Kelly’s ratio
Kelly’s Ratio was formulated by Kelly and is computed by dividing sodium ion concentration versus calcium and magnesium ion concentrations [86]
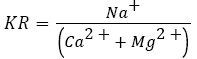
where, concentrations of all ions were expressed in meq/l. Waters with a KR value <1 are regarded suitable for irrigation, while those with higher values are considered unsuitable. Ramesh and Elango reported that water having Kelly’s ratio less than 1 is suitable for irrigation, whereas those with a ratio more than 3 are unsuitable for irrigation [49].
Patel and Vadodaria calculated the Kelly’s ratio while ascertaining the water quality in Mehsana district, Gujarat which varied from 0.09 to 4.38 epm and showed that only 30 percent samples were safe for irrigation purpose [87]. Shah and Mistry determined the water quality in Vadodara District, Gujarat, India and found that for pre-monsoon groundwater samples 33.3% Kelley’s ratio (KR) values were less than 1 and indicate good quality water for irrigation purpose while 66.67% had more than 1 suggesting the unsuitability of water quality for irrigation. However, for post monsoon samples 50% Kelley’s ratio (KR) values were less than 1 and 50% more than 1 [48].
Hardness
Determination of water hardness is a utilitarian test to evaluate quality of water for domestic, agricultural and industrial uses [79]. The hardness of water is generally caused by calcium and magnesium. However, total hardness of water can be classified into two types, i.e., temporary and permanent hardness. Since, the temporary hardness can almost be removed by boiling the water; nevertheless, the permanent hardness can be removed by boiling. Total hardness is the summation of temporary and permanent hardness. The hardness of water suggests the nature of the geological formations with which it has been in contact [29,64]. The total hardness (as CaCO3) of water samples can be calculated using the following equation,
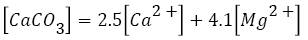
Sawyer and McCarty (1967) classified water that contains <75 mg l−1 CaCO3 as soft, 75-150 mg l−1 CaCO3 as moderately hard, 150-300 mg/l CaCO3 as hard and >300 mg/l CaCO3 as very hard.
Aghazadeh and Mogaddam analysed groundwater quality of Oshnavieh plain in Iran and concluded that the groundwater in the area was generally fresh and hard to very hard [85]. Rahman et al. reported the status of groundwater quality in Kurigram District Bangladesh and concluded that groundwater was moderately hard which indicated that it was also good in quality for irrigation purposes but not suitable for some industrial purposes especially for food making industries [88]. Peseyie and Rao assessed the quality of groundwater in Dimapur, Nagaland and reported that 94.34% of the samples come under acceptable limit and 5.66% within permissible limit with respect to total hardness [16].
Chloro-Alkaline Indices (CAI)
Determining changes in chemical composition of groundwater during its travel through the soil is essential [20,89]. To regulate the dissolution of unwanted elements in water is impractical during the subsurface movement but it is important to identify the different changes experienced by the water throughout the motion [90]. Schoeller studied the ion exchange between the groundwater and its environment during the travel though the soil [91]. The Chloroalkaline indices (1 and 2) used in the base exchange are computed by the following equations:
I) Chloro-Alkaline Index 1
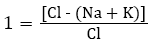
II) Chloro-Alkaline Index 2
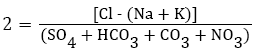
The exchange is identified as direct if there is ion exchange of Na and K from water with Mg and Ca in the rocks and the indices are negative. On the contrary, if the exchange is reverse then the exchange is known as indirect with positive indices and reported that 80% and 59% water samples in Cuddapah district of Andhra Pradesh showed positive ratio of chloro-alkaline indices in the post and pre-monsoon seasons, respectively, whereas 20% and 41% of the corresponding seasons exhibited negative ratios illustrating the type of base exchange [30]. Aghazadeh and Mogaddam reported that 26% water samples showed negative and 74% showed positive chloro-alkaline indices in Oshnavieh area of northwest Iran [85].
Saturation Index (SI)
The degree of equilibrium between water and minerals is assessed by the saturation indices. The alterations in saturation sate are valuable in deciding various stages of hydrochemical evolution and assist in identifying the geochemical reactions that are crucial in checking chemistry of water [85,92-94]. The saturation index of the mineral is calculated from the equation,
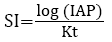
where, IAP is the ion activity product of dissociated chemical species in solution, Kt is equilibrium solubility product for the chemical involved at the sample temperature. If water is precisely saturated with the dissolved mineral, SI is equal to zero [95]. If the SI is below zero, the groundwater is considered as undersaturated regarding any mineral thereby revealing the quality of water from a formation with inadequate quantity of mineral for solution or brief residence time. On the contrary, SI greater than zero indicates that groundwater is supersaturated regarding specific mineral phase and consequently unable to dissolve more of a mineral. Therefore, this index suggests groundwater discharging from an aquifer carrying sufficient amount of mineral with enough residence time to attain equilibrium. However, super saturation may also be developed by other causes like incongruent dissolution, common ion effect, evaporation, rapid increase in temperature and CO2 exsolution [85,93,96].
Jalali calculated the mineral saturation index of groundwater by computer program PHREEQC and found that nearly 34% of samples were oversaturated with respect to calcite and dolomite [95]. However, water samples were undersaturated with respect to sulphur bearing minerals (gypsum and anhydrite) in addition to other mineral phases which might control NaCl in the aquifer. Similarly, Aghazadeh and Mogaddam calculated the saturation index by PHREEQC which showed that nearly all the water samples were saturated or undersaturated with respect to carbonate minerals and undersaturated with respect to sulphate minerals [85].
Evaluation or supervising ground water quality for irrigation purposes is of paramount importance in semi-arid and arid regions of globe, particularly in the developing countries like India owing to burgeoning population, expansion of irrigated farming and mushroom growth of industrial settings. The groundwater contamination due to salinization is major issue which can be triggered by various processes, viz., intrusion of seawater, pollution by agrochemicals, geogenic contamination and salinization induced by irrigation. Several indices mentioned above play a pivotal role in classification and assessment of groundwater quality. Since groundwater is a precious resource, therefore, there is a need to preserve and protect this valuable resource by following preventive measures to control the contamination.