
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Market Analysis - (2020)
The research report on Global Clinical Trials Market 2019 keenly analyzes significant features of the industry. The analysis servers market size, latest trends, drivers, threats, opportunities, as well as key market segments. It is based on past data and present market needs. Also, involve distinct business approaches accepted by the decision makers. Those intensify growth and make a remarkable stand in the industry. The Clinical Trials market will grow with a significant CAGR between 2019 to 2028. The report segregates the complete market on the basis of key players, geographical areas, and segments.
Increasing demand for new medical equipments and medicines among end users, coupled with growing investment for research and development activities for development of effective medicines are major factors driving growth of the global clinical trials market. In addition, increasing number of individuals suffering from chronic diseases as well as changing conditions and nature of certain types of chronic diseases is another factor anticipated to support growth of the global clinical trials market to significant extent.
The study includes basic information about the product such as Clinical Trials scope, segmentation, outlook. Likewise, it includes supply-demand static, investment feasibleness, and factors that constrain the growth of an industry. Especially, it offers product demand, yearly revenue and growth facet of the industry.
The key players operating in the global clinical trial management market include Oracle, Medidata Solutions, Parexel, BioClinica, Inc., Bio-Optronics, IBM, MedNet Solutions, Veeva Systems, Forte Research Systems, and Merge Healthcare Incorporated.
Other prominent players in the value chain include Mednet Solutions, Arisglobal, eClinForce Inc., DZS Software Solutions, DSG, Inc., Guger Technologies Inc., ICON, Plc., ChemWare Inc., and iWeb Technologies Limited.
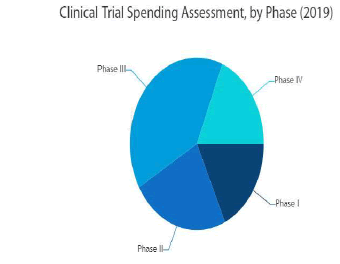
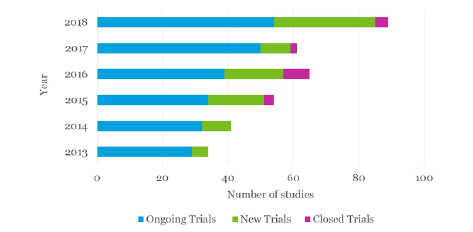
The clinical trials market is projected to arrive at USD 1.76 billion by 2025, from USD 1.04 billion in 2017 growing at a CAGR of 6.7 percent from 2018 to 2025. An upcoming market report contains data for historic year 2016, and the base year of calculation is 2017.
Data Bridge Market Research has announced the availability of Clinical Trials Market Outlook to 2025. The global industry report, which covers key players such as PAREXEL, LabCorp, ICON plc, Novo Nordisk, and Covance, provides insights, market dimensions and evaluations for the period from 2018 to 2025.
The research study provides far-reaching investigation of numerous global clinical trials industry segments in terms of applications, product components and services and geographical regions. The market report covers data points for 28 countries and addresses the global perspective of clinical trials industry with regional splits into North America, Europe, China, Japan, Southeast Asia, India, Asia Pacific and Middle East.
According to Data Bridge, the global clinical trials market is highly fragmented. Major players have utilized various strategies, including new product launches, expansions, agreements, joint ventures, partnerships, acquisitions and others to increase their impact in this market.
Data bridge also analyzed major drivers in the market in order to produce the report. They include demand for clinical trials in emerging markets, high R&D spending of the pharmaceutical industry, increasing prevalence of diseases, focus on rare diseases and multiple orphan drugs in pipeline, lack of skilled clinical research workforce, regulatory quality in emerging markets and stringent regulations for patient enrollment.
The global clinical trials market is segmented based on phase, design and geographical segments, Data bridge explained. On the basis of phase, the market is further classified into Phase I, Phase II, Phase III and Phase IV. On the basis of design, the market is segmented into treatment studies and observational studies. Treatment studies are further sub-segmented into randomized control trial, adaptive clinical trial and non-randomized control trial. The observational studies segment is further sub-segmented into cohort study, case control study, cross sectional study and ecological study.
Data collection and base year analysis are performed using data collection modules with large sample sizes. The market data is analyzed and forecast utilizing market statistical and coherent models. In addition, market share analysis and key trend analysis are critical factors in the market report.
Market Research Report Summary
Global Clinical Trial Management Systems (CTMS) Market Size, Status and Forecast 2019-2025 report is published on May 13, 2019 and has 98 pages in it. This market research report provides information about Healthcare, Clinical Trials, Pharma & Healthcare industry. It covers Global regional market data and forecasts. It is priced starting at USD 3,900.00 for Single User License (PDF) which allows one person to use this report.
The New-Age Clinical Trials Are ‘Web’ Based
Biopharmaceutical companies are focused on implementing digital health in the clinical trials and operations. It is highly likely that pilot projects and investments to validate digital measures will continue to rise, abreast the uptake of patient centricity to empower convenient and personalized approaches. The initial years of the ‘digitalization era’ also left some major imprints on the clinical trials landscape. Pzifer has already started focusing on new launches of web-based clinical trial outreach platforms, and other companies followed suit. Such efforts mark a new milestone for the global clinical trials space and fine tune the way clinical trials were conducted.Key Benefits for Stakeholders:-
This report entails a detailed quantitative analysis of the current market trends from 2016 to 2023 to identify the prevailing opportunities.
Market estimations are based on comprehensive analysis of the key developments in the industry.
The global market is comprehensively analyzed with respect to product, delivery mode, component, end user, and region.
In-depth analysis based on geography assists in understanding the regional market to assist in strategic business planning.
The development strategies adopted by key manufacturers are enlisted to understand the competitive scenario of the market.
Published: 28-Dec-2020
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.