Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Review Article - (2022)Volume 13, Issue 3
Sjogren’s Syndrome (SS) is a chronic and progressive multisystem autoimmune disease typically managed by rheumatologists, and diagnostic delays are common, which are due in large part to the non-specific and variable nature of SS symptoms and the slow progression of disease. This article reviews current understanding of the clinical manifestations, diagnosis and treatment of sjogren’s syndrome and its attendant ocular manifestation. sjogren’s syndrome is a chronic inflammatory disorder of the exocrine glands with multiple non exocrine features, and it is found predominantly in middle-aged women but exists throughout the population. The diagnosis of sjogren’s syndrome can be challenging because the cardinal sicca symptoms may be subclinical or attributed to other causes, such as medications or aging. Differential diagnosis of sjogren’s syndrome can be confounded by the multiple exocrine manifestations in the mouth, eyes, ears, nose, skin, vagina, and respiratory and gastrointestinal tracts. Characteristics of SS are dry eye and dry mouth, which are typically the earliest presenting complaints, eye care clinicians such as the optometrists and dental professionals are often the first point of medical contact and can provide critical collaboration with rheumatologists to facilitate both timely diagnosis and ongoing care of patients with SS. Current diagnostic criteria advocated by the American College of Rheumatology (ACR) are predicated on the presence of signs/symptoms suggestive of SS along with at least two objective factors such as traditional biomarker positivity, salivary gland biopsy findings, and/or presence of keratoconjunctivitis sicca. Timely diagnosis of SS requires appropriate clinical vigilance for potential SS symptoms, referral and collaborative communication among rheumatology, optometrists, ophthalmology, and oral care professions. Furthermore optometrists can now identify sjogren’s patients earlier in their dry eye population with a new advanced diagnostic test sjo, the laboratory test designed for early detection of sjogren’s syndrome, has been available from Nicox for use by eye care professionals since November, 2013. Optometrists have a role to play in the management of ocular manifestations of sjogren’s syndrome. Such symptoms can lead to discomfort, blurred vision, and visual fatigue if not attended to immediately. For people with immune system disorder, inflammation of tear-secreting glands reduces tear production, resulting in chronic dry eyes. Prescription eye drops such as Tears Naturale, Cyclosporin (Restasis) or Lifetegrast (Xiidra) may be recommended by the eye doctor if the patient has moderate to severe dry eyes.
Sjogren’s Syndrome (SS); Keratoconjunctivitis sicca; Gastrointestinal tract; Nicox
Sjogren’s Syndrome (SS) is a chronic inflammatory disease characterized by lymphocytic infiltration of the exocrine glands. Keratoconjunctivitis Sicca (KCS) (dry eyes) and xerostomia (dry mouth) are the hallmark of SS. However, the exocrine involvement in SS extends beyond the lacrimal and salivary glands; it includes the entire exocrine system of the Gastro Intestinal (GI) system, the respiratory system (the nose, sinuses and lungs), the throat, ears, skin and vagina. There are two forms of SS, primary and secondary. In primary SS, the inflammatory process is limited to the exocrine glands, while in secondary SS the exocrine involvement is associated with other connective tissue disease or autoimmune disease, such as rheumatoid arthritis or lupus erythematosus. Both primary and secondary SS may be associated with other, seemingly unrelated, diseases that are often seen in the thyroid, liver, gallbladder, pancreas, kidneys, and the musculoskeletal, vascular and nervous systems [1]. Approximately a third of SS patient’s exhibit systemic non-exocrine involvement [2]. The underlying biochemical and immunological basis for the variation in the clinical presentation is not known. A few studies have suggested that early onset of SS (<35 years of age) is associated with more severe systemic and immunological manifestations than later onset of the disease [3] but this distinction in the clinical manifestations between older and younger patients is controversial [4] (Figure 1).
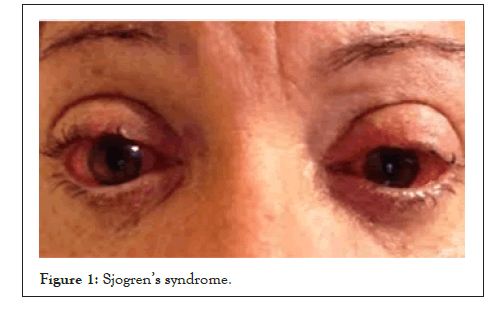
Figure 1: Sjogren’s syndrome.
Problem statement
Although SS may be mild and subclinical, serious complications, including interstitial pneumonitis, nephritis and hearing loss, may arise [5]. Most noteworthy, perhaps, is that SS patients have a greatly increased (as much as 44-fold) risk for malignant lymphoma [6]. In a study of 723 consecutive patients with primary SS, one in five deaths was attributable to lymphoma [7]. In another study of 55 SS cases, Zufferey and colleagues reported that 9% of the patients developed low-grade B-cell lymphoma over a period of 12 years (mean 6.5 years); two of them were in the lymph nodes, one in the parotid, one in the lacrimal, and one in the lungs [8]. They also suggested that patients with systemic manifestations appear to be at higher risk for malignant transformation irrespective of the presence or absence of immunological serum markers such as mixed cryoglobulinemia.
Owing to the broad spectrum of the multisystem involvement in SS, management of the disease often requires a multidisciplinary approach that includes a rheumatologist, dentist, primary care physician, ophthalmologist, gynecologist, gastroenterologist, pulmonogist, otorhinolaryngologist and dermatologist. This article reviews current understanding of the multisystem nature of the clinical manifestations, diagnosis and treatment of SS.
Prevalence
The heterogeneity of the available prevalence and incidence data for sjogren’s syndrome is explained by differences in study design and classification criteria. SS is most prevalent in women in their fourth and fifth decades with a female: male ratio of approximately 9:1 [9]. It is considered the second most common autoimmune disease next to rheumatoid arthritis, with an estimated prevalence of 0.5–5% of the population [9]. An estimated 2–4 million individuals in the USA have SS [10], but the majority of them remain undiagnosed. SS may also affect children; there are approximately 140 cases of SS in children in the literature. Unlike adult onset, the most common symptom of childhood SS is not sicca symptoms, but recurrent salivary gland infections (mostly parotitis) [8]. It has been suggested that some of the patients who are diagnosed with SS in adulthood might have experienced the early manifestations during their childhood. The global prevalence calculated for the rarer pSS is 61 per 100000 inhabitants, with the highest prevalence encountered in Europe [11]. Women develop sjogren’s syndrome significantly more frequently than men; the sex difference ranges between 9:1 to 19:1. Mean age at time of first diagnosis of pSS is 56 years, with another peak occurring between 20 and 40 years. However, first symptoms may occur years before diagnosis. As yet, there is a lack of reliable epidemiological data for Germany. The overall prevalence of sjogren’s syndrome, including the more common secondary form of the disease, is assumed to be at least 0.4% [12].
Clinical manifestation: According to the largest cohort published so far, sicca symptoms are the most common manifestation of sjogren’s syndrome, with up to 98% of cases [13]. Patients with Keratoconjunctivitis Sicca (KCS) complain about foreign-body sensation, burning or soreness of the eyes and increased sensitivity to light. Marked xerostomia as a sign of stomatitis sicca presents clinically as difficulties when talking for extended periods of time and while chewing or in salivating dry food (Figure 2).
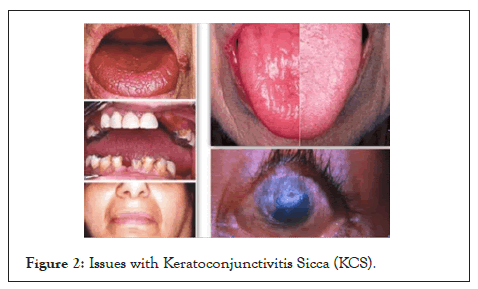
Figure 2: Issues with Keratoconjunctivitis Sicca (KCS).
Compared with the general population, the prevalence of dental caries and early tooth loss is about twice as high in patients with sjogren’s syndrome and their oral health-related quality of life is significantly reduced. Recurrent oral infections with Candida albicans occur 10 times more frequently than in the general population [14]. On the other hand, sicca symptoms are commonly reported with advancing age and polypharmacy. Approximately 5% to 35% of the general population suffer from dry eyes [15], and approximately 20% of dental patients experience dry mouth [14] (Figures 3A and 3B).
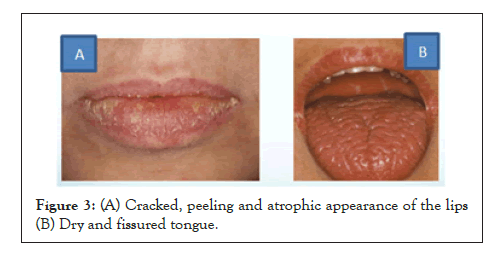
Figure 3: (A) Cracked, peeling and atrophic appearance of the lips (B) Dry and fissured tongue.
Thus, a thorough medical history, including medications, and physical examination, followed by special function tests, is crucial for the interpretation of these complaints. Table 1 lists the differential diagnoses for glandular complaints. In addition, attention should be paid to other sicca symptoms, such as dry cough in tracheobronchitis sicca or sicca symptoms in the nasopharynx or genital tract, manifesting as an increased susceptibility to infection or as dyspareunia.
| Dry eye/keratoconjunctivitis sicca | |||
|---|---|---|---|
| Normal tear production (Schirmer's test unremarkable) | Reduced tear production (abnormal Schirmer's test) | ||
| Environmental factors (air conditioning, smoking, computer work)-Meibomian gland dysfunction, rosacea-Contact lenses-Corneal hypoesthesia after LASIK surgery, along with diabetes-Incomplete lid closure | Drug-induced: Anticholinergics, antihistamines, tricyclic antidepressants, diuretics-Age-related menopause-Status post head/neck radiation-Chronic viral infections (HCV, HIV)-Sarcoidosis, lymphoma-Sjogren's syndrome (primary and secondary)-IgG4-related disease | ||
| Xerostomia | |||
| Drug-induced: anticholinergics, antihistamines, tricyclic antidepressants, diuretics, antihypertensive drugs, among others-Anxiety disorder, endogenous depression, fibromyalgia, bulimia/anorexia-Status post head/neck radiation-Systemic disease (sarcoidosis, amyloidosis, HCV, HIV) | |||
| Parotid swelling | |||
| Mainly unilateral | Mainly bilateral | ||
| Acute: | Bacterial infection, actinomycosis, mechanical obstruction by salivary duct stones | Acute: | Viral infection (mumps, EBV, CMV) |
| Chronic: | Chronic sialadenitis, neoplasia (pleomorphic adenoma of the parotid gland) | Chronic: | Chronic infections (HCV, HIV), diabetes mellitus, alcoholism, anorexia, amyloidosis, IgG4-related disease, hyperlipoproteinemia |
Note: CMV: Cytomegalovirus; EBV: Epstein-Barr Virus; HCV: Hepatitis-C Virus; LASIK surgery: Laser in-situ keratomileusis
Table 1: Differential diagnosis of glandular manifestations.
Up to 34% of patients with Sjögren’s syndrome report episodic or chronic, typically bilateral swelling of the parotid glands [16]. Here, it is essential to exclude malignant Non-Hodgkin Lymphoma (NHL) of B-cell lineage which occurs in about 5% of patients with pSS [17] who are at a significantly increased risk of developing NHL compared with the general population (Risk Ratio (RR): 13.7).
Key predictors for the development of NHL include low complement levels (RR: 8.3), cryoglobulinemia (RR: 6.8), lymphadenopathy (RR: 3.7), histological finding of ectopic germinal center-like structures, permanent swelling of parotid gland, and skin vasculitis [8]. These patients belong to a high-risk group and require monitoring at closer intervals and, if necessary, further diagnostic investigations, such as chest radiography and abdominal ultrasound; however, valid recommendations for lymphoma screening are not available.
The most common extraglandular manifestations are arthralgia and a usually non-erosive polyarthritis which occur in approximately 50% of patients [18]. Pulmonary involvement beyond the sicca complex typically manifests as interstitial lung disease or follicular bronchiolitis, normally after many years of disease activity (9-12%) [9,10]. About 10% of patients have cutaneous lesions, the majority in form of a vasculitis with involvement of small and medium vessels of the lower limbs. In addition, other less common skin manifestations may occur, such as annular erythema, urticarial vasculitis, or hypergammaglobulinemic purpura [18]. Renal involvement, which is found in approximately 5% of patients, is usually associated with tubulointerstitial changes which frequently go along with distal renal tubular acidosis (RTA type 1) with hypokalemic muscular hypotonia; glomerulonephritis is rare in patients with pSS [4,18].
Also of clinical relevance is the involvement of the peripheral nervous system, especially later in the course of the disease, typically manifesting as sensory neuropathy (10-25%) [9,11]. Rarer and more challenging to identify are CNS manifestations; for example, the differential diagnosis of multifocal CNS lesions on MRI includes multiple sclerosis lesions which are difficult to distinguish from pSS lesions [19]. In this context, the coexistence of pSS with Neuromyelitis Optica Spectrum Disorders (NMOSD), which is characterized by autoantibodies to aquaporin-4, is of importance [20]. If patients test positive for this antibody, this is of great differential therapeutic significance.
By contrast, nonspecific complaints such as fatigue and diffuse pain are more difficult to evaluate. However, fatigue is the symptom experienced as most distressing by the patient, determining physician visit frequency, quality of life as well as fitness for work. Other conditions in the differential diagnosis of fatigue, such as hypothyroidism, anemia and sleep disorders, should be excluded and difficulties in coping with the disease should also be taken into account.
Anti-Ro/SSA and anti-La/SSB-positive women desiring to have children require special counseling. Placental transmission of these antibodies can cause inflammation with subsequent sclerosis of the Atrio-Ventricular (AV) node which carries the risk of the fetus developing a congenital heart block. In 80% of cases, complete irreversible heart block occurs and in 20% fetal mortality is significantly increased [21]. Weekly ultrasonographic monitoring of the cardiac rhythm of the fetus between 16 and 31 weeks’ gestation is essential for both prognostic evaluation and management.
Signs and symptoms of SS vary with the nature of glandular and extraglandular involvement. Classically, dry eyes and dry mouth are the most predominant features of primary SS. Other exocrine and non-exocrine manifestations may include the respiratory and GI systems, ears, skin, vagina, kidneys, and vascular, musculoskeletal and nervous systems (Table 2). The combined symptoms of the exocrine and non-exocrine manifestations are useful for the diagnosis of SS [22].
| Exocrine | Non-exocrine |
|---|---|
| Oral | Musculoskeletal |
| Dry mouth | Arthritis |
| Sore mouth | Myositis |
| Candidiasis | Fibromyalgia |
| Rampant den | Arthralgias |
| Difficulty in chewing | Polyarticular arthropathy |
| Difficulty in swallowing | |
| Change in taste | |
| Ocular | Thyroid gland disorders |
| Dry eyes | Thyroiditis |
| Ocular discharge | Hypothyroidism |
| Itchy eyes | |
| Sandy feeling | |
| Foreign-body sensation | |
| Intolerance of light | |
| Otic | Neurological and psychological |
| Hearing loss | Peripheral neuropathy |
| Otitis externa sicca | Depression |
| Respiratory | Hematological |
| Dry nose | Neutropenia, anemia, thrombocytopenia |
| Recurrent sinusitis | Hypergammaglobulinenia |
| Bleeding nose | Pseudolymphoma |
| Laryngitis | Myeloma |
| Chronic cough due to dryness | |
| Recurrent bronchitis | |
| Interstitial | |
| Gastric | Renal |
| Hyperacity | Tubular-interstitial nephritis |
| Dysphagia | Glomerulonephritis |
| Atrophic gastritis | |
| Diarrhea | |
| Constipation | |
| Cholangitis | |
| Pancreatitis | |
| Vagina | Malignancy |
| Dry vagina | Lymphoma |
| Vaginal burning | Non-Hodgkin’s Lymphoma |
| Recurrent candidiasis | |
| Dyspareunia | |
| Skin and Vascular | |
| Dry Skin | |
| Skin rash | |
| Burning Skin | |
| Raynaud’s syndrome | |
| Vasculitis | |
| Purpura |
Table 2: Summary of exocrine and non-exocrine manifestation.
Respiratory involvement
Although it has been established that respiratory involvement can occur in patients with SS, the prevalence of pulmonary comorbidity is still controversial and has been reported to range from 9%–75% [23]. This wide variation is in part due to the lack of standardized diagnostic criteria and failure of the studies to consistently differentiate primary and secondary SS. Nonetheless, SS appears to affect the entire respiratory tract, including the pleura and mediastinum, and has been linked with diffuse interstitial lung disease [23], atelectasis and bronchiectasis [21], and pulmonary hypertension. In a study of 36 patients with primary SS, the most common respiratory tract finding was diffuse interstitial lung disease (25%), followed by smallairway disease (22%), upper-respiratory tract dryness (17%) and obstruction of large airways (8%) [24]. Interstitial pathology seen in patients who underwent trans-bronchial lung biopsy included dense lymphocytic infiltrates and interstitial fibrosis.
Interstitial lung disease can be categorized as lymphocytic interstitial pneumonitis, pulmonary fibrosis or lymphoma. Also characterized by pulmonary infiltration, pseudolymphoma responds well to corticosteroids, but can advance to lymphoma. Pulmonary fibrosis, present in as many as 10% of patients with primary SS [24], may result from prolonged lymphocytic interstitial infiltration. It was once believed that even secondary SS, as seen in rheumatoid arthritis, put patients at increased risk of bronchiectasis, respiratory infections and airway disease [22,24]. However, it has been suggested that in most patients with secondary SS, respiratory involvement is due to rheumatic disease [24].
Loss of mucus secretion in the bronchial tree may result in xerotrachea, chronic bronchitis or small-airway disease. Xerotrachea occurs in approximately a fifth of patients with primary SS [25], and approximately 40–60% of SS patients have hyper-responsive airways as a result of histological abnormalities in bronchi and bronchioles [23]. Although, often asymptomatic diffuse lung disease usually presents with dry cough, sometimes characterized as unrelenting, due to xerotrachea [23], or dyspnea; these are common complaints of patients with SS. Objective clinical findings may include crackles heard on auscultation, reticular abnormalities and interstitial-like patterns revealed by chest radiography, and bronchial or peribronchial thickening revealed by computed tomography [26]. Transbronchial biopsies frequently expose bronchiolar lymphoid infiltrates and follicular bronchiolitis. Lymphadenopathy or nodules should be investigated as possible indications of lymphoma [27].
Otic manifestations
The most severe otic manifestation of SS is hearing loss. In a study of 30 patients with SS who underwent audiometric testing, 14 (46%) exhibited sensorineural hearing loss [28]. Hearing loss in patients with SS may be caused by defective production of the permeability barrier of the external acoustic meatus and sensory impairment from nervous system involvement. Otitis externa sicca and fibrotizing external otitis are other complications of SS marked by defects in lamellar body secretion and the production of the permeability barrier [28].
Gastrointestinal and hepatobiliary
Dryness of the pharynx and esophagus often results in dysphagia. However, it is important to exclude other causes of dysphagia such as muscular or neurological diseases and tumors. Chronic atrophic gastritis and lymphocytic infiltrates similar to those in minor salivary glands appear in biopsies of gastric mucosa [29]. Hypopepsinogenemia, elevated serum gastrin levels, low serum vitamin B12 concentrations and antibodies to parietal cells are also seen [30].
Inflammation and destruction of the mucus-secreting glands of the GI tract in Figure 4 and mucosal atrophy have been reported as common findings in patients with primary SS. Mucosal atrophy can affect the entire length of the GI tract and may be associated with upper esophageal webs, dysmotility and achalasia, vasculitis, esophageal candidiasis, and reflux disease. The mechanisms proposed to explain this symptom include the decreased production of saliva, esophageal dysmotility, upper esophageal webs and achalasia [31]. Mucosal atrophy can also be seen throughout the entire length of the esophagus [32]. Chronic atrophic gastritis commonly occurs in SS [31]. Inflammatory changes in the intestines, related to an immune mechanism, have been described and there is also an increased risk for the development of lymphoma, such as mucosa-associated lymphoid tissue lymphomas within the GI tract [33] (Figure 4). The figure shows erythema of the mucosa of the oral cavity (A) esophagus (B), and colon (C). Evaluation for malignancy is important if endoscopic studies are performed for symptoms of abdominal fullness or epigastric pain.
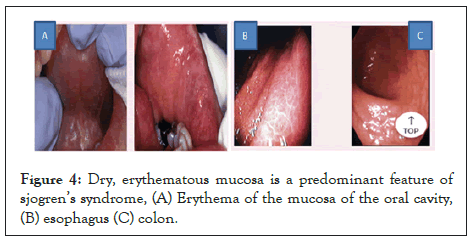
Figure 4: Dry, erythematous mucosa is a predominant feature of sjogren’s syndrome, (A) Erythema of the mucosa of the oral cavity, (B) esophagus (C) colon.
Liver involvement was thought to be uncommon (5%) and subclinical, signaling its presence with elevated serum levels of liver enzymes or antimitochondrial antibodies [34]. Biopsy may show features of stage I Primary Biliary Cirrhosis (PBC), and sicca symptoms are seen in 50%–80% of these patients [35]. More recently, liver involvement has been found to be a common complication that merits routine investigation in patients with primary SS [36]. In a 5-year follow-up study of patients with SS, Csepregi and colleagues found that high titers of smooth muscle antibodies and anti-mitochondrial antibody can be an indicator for autoimmune hepatic disease and PBC [37]. It has been proposed that the initiating event leading to liver disease starts in the epithelial lining of bile ducts within the liver. Therefore, it is advisable to screen SS patients for these antibodies. It was seen more frequently in patients who also had evidence of lung, kidney and hematological abnormalities. Patients with evidence of liver involvement should be monitored by a hepatologist.
PBC and chronic active hepatitis of the autoimmune type are associated with SS, while HCV infection is currently excluded in classification criteria. A Swedish study of 45 primary SS patients with sicca symptoms found that 27% had abnormal liver enzyme levels [38]. Further evaluation showed that four (9%) of these patients with primary SS also had PBC, and two (4%) had chronic active autoimmune hepatitis.
Chronic pancreatitis and sclerosing cholangitis may result from the eventual fibrosis of the exocrine glands and pancreas in association with the biliary duct system in some cases of SS [39]. Others have reported cases of acute and chronic pancreatitis in association with SS [40]. One study demonstrated an immunogenic response to a protein isolated from the pancreas in patients with idiopathic chronic pancreatitis and SS [40]. On radiography or autopsy, pancreatic abnormalities, such as mononuclear-cell infiltration and loss of reticulin pattern, pancreatic calcification and fat necrosis, have been revealed [3].
Obstetric and gynecological
There is scant information regarding the effects of SS on the female reproductive tract, and problems potentially related to SS may be missed. Vaginal and vulval dryness are common exocrine manifestations that can result in dyspareunia and pruritus [41]. Unfortunately, like other sicca symptoms, these are often attributed to ‘natural aging’ or menopause. Other pelvic diseases, such as vaginal infection, should be ruled out as the specific cause for dryness is sought. There are special obstetric considerations for patients with SS. Maternal autoimmune disease results in higher rates of spontaneous abortion and fetal death. Primary SS in the mother has been linked with congenital heart block [42]. Anti-Ro and anti-La antibodies have been shown to cross the placenta and cross-react with the conducting system of the fetal heart [42].
Cutaneous and vascular
Reduced exocrine secretions in the skin result in dryness [43]. Heterogeneous skin lesions that are seen include purpura, urticaria, and annular lesions. Approximately a third of SS patients also have Raynaud’s syndrome, which often long precedes sicca manifestations and disappears or decreases in frequency in approximately half of the affected patients [44].
Vasculitis is limited to the skin in approximately a third of patients with primary SS, appearing as palpable or nonpalpable purpura or lesions similar to urticaria [30] (Figure 5). Leukocytoclastic vasculitis is the most common histological sign. Systemic necrotizing vasculitis, although uncommon, may occur in patients with primary SS. It may occur in association with mixed cryoglobulinemia and takes the form of ulcerative skin lesions, digital gangrene, mono-neuritis multiplex, myositis and glomerulonephritis [30]. Occasionally, systemic vasculitis is seen with visceral involvement affecting the kidneys, lungs and GI tract [27]. Patients with secondary SS, which is very often associated with Systemic Lupus Erythematosus (SLE), may exhibit the myriad well-characterized skin manifestations of SLE (Figure 5). The figure shows vasculitis of the legs (A), necrotizing vasculitis of the legs (B), and vasculitis of the colon (C).
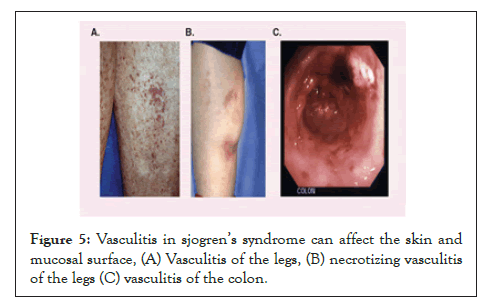
Figure 5: Vasculitis in sjogren’s syndrome can affect the skin and mucosal surface, (A) Vasculitis of the legs, (B) necrotizing vasculitis of the legs (C) vasculitis of the colon.
Musculoskeletal: Secondary SS is also very often associated with rheumatoid arthritis in Figure 6. However, even patients with primary SS who have significant systemic involvement report fatigue, general malaise, low-grade fever, myalgias and arthralgias. They may experience brief episodes of a lupus-like non-erosive arthritis that is sometimes followed by Jaccoud’s arthropathy. However, serum levels of muscle enzymes are usually normal or only mildly elevated, unless severe myositis occurs [23]. Arthritis and arthralgia may be present in 60% of patients with primary SS in the form of intermittent polyarticular arthropathy, affecting the small joints, sometimes asymmetrically [45]. Joint deformity and mild erosions are unusual. Myalgias occur frequently in primary SS despite a low incidence of inflammatory muscle disease (Figure 6). The figure shows rheumatoid arthritis (A) and scleroderma (B) in patients with secondary sjogren’s syndrome.
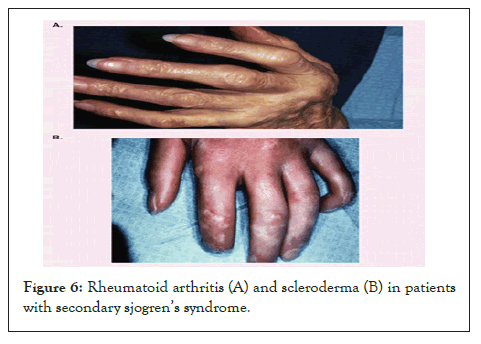
Figure 6: Rheumatoid arthritis (A) and scleroderma (B) in patients with secondary sjogren’s syndrome.
Cardiovascular
Cardiovascular manifestations may include parasympathetic dysfunction. Abnormal heart rate responses to both the Valsalva maneuver and forced respiration were seen in 15% of patients with primary SS in one study, although no signs of sympathetic dysfunction were observed [23,27]. In a subsequent study using power spectral analysis techniques, investigators found no differences in parasympathetic or sympathetic function between patients with primary SS and healthy controls, although some cardiovascular differences were seen during orthostatic challenge [27]. Isolated congenital heart block has been observed in infants of mothers with primary SS; this was discussed previously in the section on obstetric and gynecological manifestations.
Renal
Renal involvement in SS arises from lymphocytic infiltration of the renal parenchyma or immune complex deposition in the glomeruli. Dysfunction, usually mild and subclinical, is not related to the age of the patient or the duration of disease. Results of the urine acidification test are abnormal in approximately 30% of SS patients, although only approximately 10% have overt renal disease [41]. Interstitial disease is the most common renal abnormality, and most of the patients with interstitial nephritis have hyposthenuria and hypokalemic hyperchloremic distal renal tubular acidosis associated with hypergammaglobulinemia [17]. Distal tubular acidosis can be subclinical or present with recurrent renal colic or hypokalemic muscular weakness. Untreated, it can lead to nephrocalcinosis, renal stones and compromised renal function [41]. Membranous or membranoproliferative immune complex glomerulonephritis associated with cryoglobulinemia and hypocomplementemia has been described in several patients with primary SS [14].
Endocrine
One in two SS patients has subclinical thyroid disease [46], with a greater incidence in primary SS than in secondary SS [47]. The histopathological findings in the thyroid glands of patients with autoimmune thyroiditis resemble those in the salivary and lacrimal glands of patients with SS. It is, therefore, not surprising that autoimmune thyroiditis is the most frequent organ-specific autoimmune disease recognized in patients with primary or secondary SS [48]. Approximately a third of patients with autoimmune thyroiditis are found to have features of SS, indicating that the two diseases may be related pathogenetically [49].
Hypothyroidism may be associated with a hypothalamic deficiency of corticotropin-releasing hormone, and some authors have speculated regarding central adrenal deficiency in SS patients. Autoantibodies to thyroid antigens and overt autoimmune thyroid disease are common in SS patients; the syndrome is considered an autoimmune epithelitis that targets polarized epithelium such as that of the thyroid [50]. The prevalence of primary SS among patients with autoimmune thyroiditis was ten-times higher than in the general population, while that of autoimmune thyroiditis was nine-times higher in patients with primary SS than in the general population [51]. In one study of endocrine disorders and immunological parameters in 43 consecutive patients with primary SS, 14 patients (33%) had thyroid disease and 13 (30%) had autoimmune thyroiditis [51].
Furthermore, the hypothalamic-pituitary-adrenal axis, as well as the thyroid axis, has been shown to be hypoactive in SS patients, through assessment of basal and stimulated adrenocorticotropin, cortisol, thyroid-stimulating hormone, and prolactin levels [52]. Hypoactivity of the stress system has been implicated in various systemic autoimmune rheumatic diseases [53]. Sex hormones also appear to play an important role in the development of autoimmune disease, most likely accounting for the strong female predominance and, in SS, the peak incidence at menopause, when sex hormones fluctuate greatly. Not only is primary SS rare in men, but serological findings in male patients have been reported to differ from those in their female counterparts [53,54].
Neurological and psychiatric
Disease of the CNS, Peripheral Nervous System (PNS), or both can develop in patients with SS. The sensory, motor and autonomic components of the PNS may be affected. Peripheral neuropathy is the most common neurological complication of primary SS, occurring in 20–30% of patients [14]. The spectrum of PNS disease resembles many types of diabetic neuropathy. Among the most common PNS manifestations of primary SS is neuropathy involving the sensory nerves to the feet and, less often, the hands, which is usually non-disabling. There is also a loss of sensory fibers that recognize position and vibratory impulses essential for maintaining awareness of limb position, posture, balance and normal gait; joint damage may result. Patients may have uncomfortable sensations of pulling or crawling that affect the trunk and extremities. Sensory neuropathy involving the extremities may be accompanied by trigeminal neuralgia. PNS involvement in SS may include carpal tunnel syndrome, caused by the pressure of inflamed tissue against the median nerve [55]. Peripheral sensory or sensorimotor polyneuropathy or mononeuritis multiplex occurs, symmetrically or asymmetrically, in 10-20% of patients with primary SS [10]. Trigeminal neuropathy was the most frequently observed neurological symptom in 21 female patients with primary SS in a Japanese study; it occurred in eight (50%) of the 16 patients with objective neurological abnormalities. CNS involvement was observed in three (14%) of the 21 patients [17].
CNS complications resembling the signs and symptoms of Multiple Sclerosis (MS) have been reported to occur in as many as 20% of patients with SS [17]. However, later studies in British and Greek populations found no CNS involvement in SS patients, nor were SS present in a sizeable study of MS patients [14]. A recent report of three cases of SS-associated myelopathy suggested that CNS disease occurs in fewer than 1% of patients with primary SS [56].
Debate regarding the incidence of severe and relapsing neurological disease in SS continues. One study found that most neurological symptoms in patients with primary SS were mild, and that in patients with secondary SS neurological symptoms were more probably caused by the underlying autoimmune rheumatic disease [48]. In another recent study autonomic nervous system dysfunction was no more prevalent in patients with primary SS than in healthy, age- and sex-matched controls [53]. The psychological effects of SS were assessed in a large community-based study in adults aged 18-75 years [41]. Standardized questionnaires evaluated psychological distress, fatigue and health status.
SS was defined by serological criteria including presence of at least one of the serum antibodies anti-Ro/SS-A, anti-La/SS-B, antinuclear antibody or rheumatoid factor; Unstimulated Salivary Flow (USF) of 0.5 ml or less in 5 min; positive Schirmer I ocular test ( ≤ 5 mm in 5 min); and questionnaire responses regarding ocular and oral symptoms. The syndrome was perceived to have a significant impact on perceived health and well-being, with higher levels of depression and anxiety found in SS patients than in those without the disorder (Figure 7).
Affective disorders developed in a majority of patients with primary SS in a study of neuropsychiatric dysfunction [17]. Patients generally presented with hysteroid dysphoric features. Several patients taking cognitive function tests showed mild memory impairment with attention and concentration deficits. A significant correlation was established between psychiatric disturbances and neurological dysfunction that suggested a possible organic basis for psychiatric dysfunction. Anxiety, depression and personality structure disorders are frequently seen and possibly associated with dysregulated stress responses [57]. Patients with primary SS scored significantly higher for ‘possible’ clinical anxiety (48%) and depression (32%) on neuropsychiatric questionnaires in comparison with healthy controls [17]. Irritability, low mood, headache, GI symptoms, and impaired concentration and memory were more frequent in patients with primary SS than in those with RA.
Other manifestations
Extreme fatigue may be the most troublesome symptom of SS; many patients spend hours in bed trying to rest or sleep and awake quite tired. Patients with SS rest or sleep on average 2 more hours a day than age-matched healthy peers [58]. Fatigue may also arise from non-restorative sleep associated with secondary fibromyalgia, depression or multiple awakenings due to dryness. Hypothyroidism, which is frequently associated with SS, although often subclinical, may partially contribute to this. Patients with more severe non exocrine impairment exhibit easy fatigue and low-grade fever [39].
Fibromyalgia, Chronic Fatigue Syndrome (CFS) and malaise are also detected with SS. Antidepressants, stress reduction and physiotherapy can be useful in managing the symptoms of fibromyalgia, which include musculoskeletal pain, point tenderness and fatigue. Fibromyalgia has been reported with relative frequency in patients with primary SS (22%) [42]. CFS is also associated with extreme fatigue and an array of physical and neuropsychological symptoms that may overlap with those of SS [42]. The differential diagnosis of these conditions is therefore challenging. Mild normochromic or normocytic anemia is common in SS [10] and an elevated erythrocyte sedimentation rate is detected in approximately 80% of patients with primary SS [10].
Lymphoproliferative disease and mortality
There is an approximately 44-times greater incidence of Non- Hodgkin’s Lymphoma (NHL) among patients with primary SS than among age-matched controls. One in five deaths in these patients is attributable to NHL [59]. In some patients, there is a progression from benign autoimmune exocrinopathy to pseudolymphoma, and in approximately 5% of patients, to lymphoma [60]. Persistent parotid gland, spleen or lymph-node enlargement is a sign of possible lymphoma. Sudden loss of autoantibodies is also suggestive of malignancy. Lymphoma can begin in the salivary glands, lymph nodes, bone marrow, thymus, liver, spleen, kidney or mucosal sites and localizes in extranodal areas. Malignancy in SS is usually B-cell NHL, but occasionally Waldenström’s macroglobulinemia. Whenever there is any suggestion of malignancy, consultation with the appropriate oncologist is indicated.
A long-term, prospective study of the impact of primary SS on morbidity and mortality found that the initial presentation determines the outcome [61]. The overall mortality of patients with primary SS compared with the general population doubled, after adjustment for age and gender, only in patients with adverse predictors. Lymphoproliferative disorders, glomerulonephritis and death were consistently associated with the presence of palpable purpura, low C4 complement levels and mixed monoclonal cryoglobulinemia, the latter two being the more important predictors. Most patients with primary SS did not have adverse predictors and had the same mortality rate as the general population.
Diagnosis
SS is underdiagnosed and often misdiagnosed, primarily due to the diversity of its clinical manifestations, which may resemble or overlap with those of a broad range of autoimmune disorders. In addition, patients do not frequently report dryness symptoms because they consider them part of normal aging, a side effect of medications, a result of mouth breathing, or a result of other causes, but rarely find them related to the onset of another symptom. Physicians, on the other hand, may not inquire regarding dryness symptoms, may consider them insignificant, or may address them individually, thereby overlooking the possibility of a generalized exocrinopathy. Moreover, different definitions and classification criteria that have been used for the diagnosis of SS present an additional challenge to physicians [39].
For these reasons, a delay of up to 10 years between the appearance of initial nonspecific symptoms and clinical diagnosis is not uncommon [62]. Although various classification criteria and definitions of SS have been proposed to standardize research, debate continues on their usefulness in clinical practice [62]. As with other rheumatic diseases, there is no single distinguishing hallmark that is diagnostic for SS. The diagnosis of SS requires satisfying a set of clinical and laboratory findings that may not always be present throughout the clinical course of the disease. Due to the importance of early diagnosis for the optimal management of SS, an expert clinician’s assessment may still be the ‘gold standard’ to define the diagnosis [48]. Diagnosis requires the presence of subjective symptoms and objective measurement of saliva and tear production, minor salivary gland biopsy, and a number of autoimmune markers. SS has a profound impact on patients’ quality of life, and screening should include an exploration of symptoms and their effect on daily life.
Despite continued advances in our understanding of the mechanisms involved in the pathogenesis of the disease, a targeted treatment of sjogren’s syndrome is not available at present. Treatment is decided on an individual basis according to disease activity and the presence and extent of extra glandular manifestations. In patients with SS, the indication for treatment is based on the underlying disease. In general, treatment should be provided by an interdisciplinary team, including family physicians, rheumatologists, Primary eye care professional (optometrist), ophthalmologists and ETN specialists, as well as dentists. Subject to the organ(s) involved and the presenting symptoms, consultation of other specialists (gynecologists, pulmonologist, neurologists, etc.) may be Cysteine required. Disease-modifying therapy is reserved for patients with extra glandular involvement. To measure systemic disease activity, the EULAR Sjogren’s Syndrome Disease Activity Index (ESSDAI) was developed and validated.
Optometrists have a role to play in the management of ocular manifestations of sjogren’s syndrome. Such symptoms can lead to discomfort, blurred vision, and visual fatigue if not attended to immediately. For people with immune system disorder, inflammation of tear-secreting glands reduces tear production, resulting in chronic dry eyes. Prescription of eye drops such Tears Naturale, Cyclosporine (Restasis) or Lifetegrast (Xiidra) may be recommended by the eye doctor if you have moderate to severe dry eyes. Herbs and vitamin supplements commonly prescribed for patients with sjoren syndrome are Cysteine, Evening Primrose (EP), Gamma-Linolenic Acid (GLA), Omega-6 Fatty Acids (OFA), and Sulfur. An attempt to treat humans with sjogren’s syndrome by raising endogenous PGE1 production by administration of essential fatty acid PGE1 precursors, Pyridoxine and Vitamin C was successful in raising the rates of tear and saliva production.
Sjogren’s syndrome is a common autoimmune disease of which the diagnosis and treatment are frequently delayed. Due to its systemic involvement, it can exhibit a wide range of clinical manifestations that contribute to confusion and delay in diagnosis. An increased awareness of SS and its many and varied manifestations encourages a more expansive approach to diagnosing this disease. The use of recently refined criteria for Journal of Oral and Maxillofacial Research (JOMR) Gomes diagnosis can assist in identifying patients with SS early. Particularly, due to the fact that there is no simple and validated test for SS diagnosis and the need for an easy, low-cost and straightforward test for the assessment of the oral component of SS, is still highly demanded. The use of all available diagnostic modalities will help to reduce the time of diagnosis and preserve the health and quality of life of patients with SS.
However optometrists can now identify sjogren’s patients earlier in their dry eye population with a new advanced diagnostic test Sjo, the laboratory test designed for early detection of sjogren’s syndrome, has been available from Nicox for use by eye care professionals since November, 2013.
Early diagnosis is essential to optimal management, but is often missed due to the potential latency and multiplicity of SS symptoms and their resemblance to those of other disorders. Thus, the expert clinician’s assessment may still be the gold standard for diagnosis. The diagnosis of SS requires evaluation of both the exocrine and the non-exocrine components of the disease. A questionnaire addressing the combined exocrine and non-exocrine symptoms provides a valuable screening tool for SS.
Here in Nigeria, optometrists should form part of the team of management especially in our teaching hospitals, specialist hospitals, general hospitals, cottage hospitals and primary healthcare centres.
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
Citation: Victor DA (2022) Advances in Clinical Optometry-Sjogren’s Syndrome. J Clin Exp Ophthalmol. 13:916
Received: 01-Mar-2022, Manuscript No. JCEO-22-16067; Editor assigned: 03-Mar-2022, Pre QC No. JCEO-22-16067 (PQ); Reviewed: 17-Mar-2022, QC No. JCEO-22-16067; Revised: 22-Mar-2022, Manuscript No. JCEO-22-16067 (R); Published: 29-Mar-2022 , DOI: 10.35248/2155-9570.22.13.916
Copyright: © 2022 Victor DA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.