
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2021)Volume 11, Issue 6
Purpose: The main purpose of the present study was to identify a volatile material that activates human Nasal Mucociliary Clearance (NMC).
Methods: Ten volatile materials were evaluated in human nasal epithelial cells (MucilAir) by measuring Ciliary Beat Frequency (CBF). The effect of orange essential oil on the chloride channel was assessed in the human intestinal epithelial cell line, T84, using the fluorescent dye N-(ethoxycarbonylmethyl)-6-methocyquinolinium bromide (MQAE) corresponding to intracellular chloride. A total of 13 individuals participated in the study, and the effect of orange essential oil on human NMC was measured using saccharine test.
Results: Treatment of spearmint Midwest rectified bergamot oil Italy bergapten free and orange essential oil significantly increased CBF in MucilAir compared with treatment of mineral oil (the control). In addition, it was observed that orange essential oil activated the chloride channel in T84 cells using MQAE. Finally, the median NMC time periods of the saccharine test were 1,000 s in the control group and 850 s in the orange essential oil group (p<0.05), indicating that inhalation of orange essential oil improves NMC in humans.
Conclusion: Our results indicated that inhalation of orange essential oil improved NMC. In addition, some volatile materials might serve to improve NMC to protect from pathogen.
Nasal mucociliary clearance; Orange essential oil; Upper respiratory tract infection; Volatile material
An infectious disease such as influenza is an important global health care concern. Potential modes of transmission of influenza virus include direct contact with virus-contaminated objects (fomites), exposure to droplets in the air from coughing or sneezing, and inhalation of infectious aerosols [1]. Hand hygiene is important to prevent from direct contact, and surgical mask is known to be useful to protect from virus-droplets. However, there is no effective way to defend against aerosols, except for ventilation of rooms, and it has been reported that Nasal Mucociliary Clearance (NMC) is one of the most important defense systems against infectious aerosols [2]. Pathogens entering the airway are flushed away into the digestive system or exhaled to the external environment in the form of sputum by NMC [3]. It has been reported that NMC decline contributes to upper respiratory tract infections such as influenza [4,5]. According to a previous study, virus injected into the nose of a dehydrated chicken with declined NMC propagated significantly when compared to chicken without declined NMC [6].
NMC occurs in the entire airway, such as in the nose, pharynx, larynx, trachea, bronchi, and bronchioles. Cilia on the airway epithelial cells move the nasal mucus regularly over the epithelial cells toward the nasopharynx, and this phenomenon is called NMC [7]. Some factors such as temperature and humidity affect NMC [8-10], and SPA bathing is demonstrated to be useful for improving NMC [11]. However, pharmaceutical drugs are the only available tools that can regulate NMC.
Some volatile materials have also been reported to possess physiological functions. Phytoncide is an aromatic volatile substance derived from trees; it contains monoterpenes such as a-pinene and limonene and has been reported to activate human natural killer cell function [12]. Lavender, which is used for aromatherapy, was demonstrated to decrease sympathetic nerve activity, and increase the skin blood flow and has effects not only on the psychological aspects but also physical [13]. In the present study, we focused on volatile materials that can activate NMC, because volatile materials could reach to the entire airway, and there is a little chance to hinder nasal care such as liquids and solids. We investigated the effects of several volatile materials on human NMC in vitro and in vivo.
The human nasal epithelial cell line consisting of primary epithelial cells, MucilAir (EP01MD), was purchased from Epithelix (Genève, Switzerland). The MucilAir primary cells were maintained according to the manufacturer's protocol. The nasal primary cells derived from healthy patients were cultured at the air-liquid interface in culture medium using cell culture inserts. The cells were maintained at 5% CO2 and 37˚C, and the fresh medium was replaced every 2-3 days.
The human intestinal epithelial cell line, T84 (EC88021101-F0), was purchased from KAC Co., Ltd. (Kyoto, Japan). T84 epithelial cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM)/Ham's F-12 with L-glutamine and phenol red (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) supplemented with 5% heat-inactivated fetal bovine serum (Biosera, Nuaillé, France) and penicillin-streptomycin solution (FUJIFILM Wako Pure Chemical Corporation). T84 cells were seeded into 96-well culture plates at a density of 104-5 cells/well, and the fresh medium was replaced every 2-3 days.
Evaluation of 10 volatile materials using MucilAir cells
The Ciliary Beat Frequency (CBF) of MucilAir cells was measured before treatment. MucilAir cells were covered with paper soaked in each volatile material (100 ppm assuming that the entire compound was volatilized), or in mineral oil (Sigma- Aldrich Japan, Tokyo, Japan) as the negative control. Subsequently, they were covered with parafilm and incubated at 5% CO2 and 37˚C for 15 min. Similarly, MucilAir cells were covered with paper soaked in mineral oil and incubated in medium with 20 μM forskolin (FSK; FUJIFILM Wako Pure Chemical Corporation) as the positive control.
Measurement of CBF
Videos of the cell surface were acquired using a CMOS highspeed camera (Digital Image Technology; DITECT, Tokyo, Japan). They were subsequently analyzed and CBF was calculated using DIPP Motion Five (DITECT). ΔCBF was calculated as the difference in CBF before and after volatile material treatment.
Measurement of chloride channel activation by MQAE fluorescence
To quantify the changes in intracellular chloride, the fluorescent dye N-(ethoxycarbonylmethyl)-6-methocyquinolinium bromide (MQAE; DOJINDO LABORATORIES, Kumamoto, Japan) was used [14,15]. 10 mM MQAE was loaded onto T84 epithelial cells overnight. These cells were washed thrice with chloride buffer (135 mM NaCl, 1 mM CaSO4, 1 mM MgSO4, 2.4 mM Na2HPO4, 0.6 mM KH2PO4, 10 mM HEPES, 10 mM glucose, 10 μM tributyltin chloride, and 5 μM nigericin), and then T84 cells were incubated for 10 minutes at 20-25˚C in 100 μL of the chloride buffer containing orange essential oil (100 ppm), FSK (10 μM), or nothing (the control). MQAE present in the cells was excited at 350 nm and the emission Fluorescence Intensity (FI) was measured at 460 nm (base line). Next, 20 μL of each buffer over T84 cells was thrown away and 20 μL of NO3 buffer (135 mM NaNO3, 1 mM CaSO4, 1 mM MgSO4, 2.4 mM Na2HPO4, 0.6 mM KH2PO4, 10 mM hepes, 10 mM glucose, 10 μM tributyltin chloride, and 5 μM nigericin) containing orange essential oil, FSK, or nothing was added to the corresponding remaining buffer. FI was rapidly measured (first measurement), and similarly, 20 μL of each buffer was transferred to 20 μL of the corresponding NO3 buffer, and FI was recorded (second and third measurements). FI was calculated as the difference between each FI and base line.
Study population
A human study was conducted at Kao Corporation (Tochigi, Japan) between April and May 2019. Ethics committee approval was obtained, and the study was conducted according to the Declaration of Helsinki. Informed consents were obtained from all the subjects.
A total of 26 male subjects between 20 and 65 years of age were included in the study. Subjects with respiratory diseases (e.g. sinusitis and asthma), nasal wounds, and history of smoking were excluded from the study. Subjects with serious illness and recent hospitalization, and those on medications were also excluded.
Study design on the effect of orange essential oil on NMC
This study comprised a cross over trial. Subjects rested for 15 min in a room, which was maitained at 20˚C and 20% relative humidity. They were instructed to sit and sniff a cotton ball soaked with orange essential oil (100 ppm assuming that the entire compound was volatilized) or an empty bottle for 10 min, and a saccharine test was subsequently conducted. Wash out period was over 3 days.
Measurement of NMC
NMC time was evaluated using the saccharine test by the same practitioner. Subjects were asked to not consume any food or drink for 1 h before the test. Saccharine tablet was placed in the nasal cavity. Patients were instructed to settle into a comfortable sitting position and not sneeze, cough, snuffle, blow nose, talk, or take a deep breath during the test [16]. The time taken from the placement of the tablet to the perception of sweet taste was recorded as the NMC time.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) software for Windows. Comparison between ΔCBF of the control and each material was performed using the t-test. Analysis of variance (ANOVA) was performed and Dunnett's post-hoc test was used for comparison between ΔFI of the control and each group. NMC time was tested using paired t-test. The statistically significant level was set at p<0.05.
MucilAir cells were exposed to the volatile materials and CBF was measured. CBF is reported to be correlated with NMC and is used as one of the indicators to assess NMC [17].ΔCBF was 1.17 ± 0.17 Hz (spearmint Midwest rectified), 2.06 ± 0.39 Hz (bergamot oil Italy bergapten free), 2.16 ± 0.77 Hz (orange essential oil), and 3.15 ± 0.46 Hz (FSK; positive control), and these ΔCBF values were significantly increased compared with that of the control (Table 1).
For studying the mechanism underlying these changes in CBF, the effect of orange essential oil, which changed ΔCBF the most, on the chloride channel, was investigated. T84 cells were exposed to orange essential oil and the ΔFI of MQAE was measured. ΔFI was -23019 ± 7397 (the control) and 22421 ± 4380 (orange essential oil) in 1 measurement, and ΔFI was 14438 ± 5392 (the control) and 53390 ± 7493 (orange essential oil) in 2 measurement. In 3 measurement, ΔFI was 37304 ± 3811 (the control) and 68534 ± 10581 (orange essential oil). ΔFI of orange essential oil significantly increased compared with that of the control in every measurement (Figure 1).
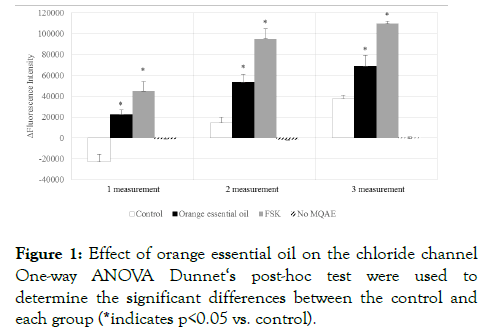
Figure 1:Effect of orange essential oil on the chloride channel One-way ANOVA Dunnet‘s post-hoc test were used to determine the significant differences between the control and each group (*indicates p<0.05 vs. control).
Finally, the saccharine test was conducted to measure human NMC [18]. A total of 26 subjects participated in this study, and 13 subjects completed the saccharine test; 11 participants did not complete this test because of their physical conditions (e.g. cold or allergy to pollen), the effects of the test treatment on runny nose, or doing prohibited matter, and 2 were dropped out of the study. The median NMC time was 1,000s in the control group and 850s in the orange essential oil group, and the NMC time of orange essential oil was significantly shorter than that of the control (Figure 2). Our findings indicated that inhalation of orange essential oil improved human NMC.
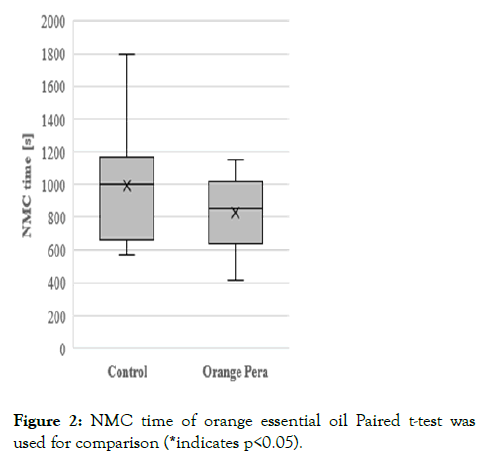
Figure 2:NMC time of orange essential oil Paired t-test was used for comparison (*indicates p<0.05).
In the present study, we observed that orange essential oil improved human NMC in vitro and in vivo and activated the chloride channel. We suggest that mucus hydration through chloride channel is one of the mechanisms for NMC activation by orange essential oil. NMC is defined mainly by the speed of ciliary movement and the viscosity of nasal mucus [3,19]. Studies on phytopharmaceuticals used to treat respiratory ailments, including rhinosinusitis and bronchitis, have reported an improved viscosity for NMC, through mucus hydration via the chloride channel [20,21]. A detailed study on the effect of orange essential oil on the airway surface liquid hydration is, however, necessary. The viscosity of nasal mucus is controlled through sympathetic and parasympathetic nerves [22]. Previous studies have shown that the inhalation of orange essential oil effectively reduced the levels of stress and anxiety [23,24]. In the present study, all subjects enrolled according to their answers to a questionnaire preferred the fragrance of orange essential oil (data not shown). Thus, a psychological change based on the preference of fragrance may be related to NMC. However, contribution of orange essential oil on the autonomic nervous system remains unclear in our study owing to the lack of negative control fragrance in the saccharin test. Further investigation of the effects of orange essential oil on the autonomic nervous system is required, which will help in understanding the main mechanism of NMC activation by orange essential oil, mucus hydration through chloride channel, and its relationship with the autonomic nervous system.
A combination of limonen, alpha-pinene and cineol was shown to increase NMC [25]. Limonen is the main component of orange essential oil; thus, we inferred that limonen in orange essential oil may lead to NMC activation. However, lemon Italy, which has almost the same amount of limonen as orange essential oil, did not increase NMC in MucilAir cells (Table 1). Therefore, an additional component might be present in orange essential oil, which may not be present in lemon Italy, and be involved in NMC activation.
| ΔCBF [Hz] | |||
|---|---|---|---|
| Control | -0.28 | ± | 0.46 |
| Lemon Italy | 0.54 | ± | 0.39 |
| Lavender extra | 0.96 | ± | 0.81 |
| Spearmint Midwest rectified | 1.17 | ± | 0.17* |
| Cedarwood Virginia | 1.19 | ± | 0.52 |
| Lime oil distillation | 1.20 | ± | 0.38 |
| Eucalyptus oil | 1.28 | ± | 0.66 |
| Peppermint Willa rectified | 1.38 | ± | 0.69 |
| Rosemary oil | 1.62 | ± | 0.98 |
| Bergamot oil Italy bergapten free | 2.06 | ± | 0.39* |
| Orange essential oil | 2.16 | ± | 0.77* |
| FSK | 3.15 | ± | 0.46* |
Table 1: ⊿CBF of the volatile materials t-test was used for comparison (*indicates p<0.05 vs. control).
In the present study, half of the participants did not complete the saccharine test, and the size of the study population was limited. Therefore, a large-scale trial conducted in seasons excluding seasonal turn and hay fever seasons is essential.
The results of the current study demonstrated that orange essential oil activates NMC. Our study indicated the potential of orange essential oil in enhancing the defense system of the body against various pathogens. Utilization of volatile materials is important for global health care and future studies on physiological functions of volatile materials could help in the betterment of human health.
Funding
This study received no funding
The authors declare that there are no conficts of interest
The study was conducted according to the guidelines of the Declaration of Helsinki on Biomedical Research Involving Human Subjects. The protocol was approved by Kao Corporation ethics committee (T201-190220). This study was also registered for University Hospital Medical Information Network (UMIN000036344).
All participants provided their written informed consent.
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Not Applicable
All authors would like to thank Dr. Suzuki (Teikyo University Medical Center) for the instruction on saccharine test. Additionally, we would like to thank Editage (www.editage.com) for English language editing.
Citation: Takada I, Miyoshi H, Mori T, Ota N (2021) Activation of Nasal Mucociliary Clearance by Orange Essential Oil. J Clin Trials. 11:486.
Received: 13-Oct-2021 Accepted: 27-Oct-2021 Published: 03-Nov-2021 , DOI: 10.35248/2167-0870.21.11.486
Copyright: © 2021 Takada I, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.