Enzyme Engineering
Open Access
ISSN: 2329-6674
ISSN: 2329-6674
Research Article - (2012) Volume 1, Issue 1
Enzymes play more and more important roles in modern food industry and attract much attention for their potential industrial applications [1]. However, as a protein, enzyme is usually limited to its activity, stability and reaction conditions in catalytic reaction. Therefore, appropriate modification to increase the activity and stability of enzyme is essential for applications, such as genetic engineering, immobilization and/or process alterations, chemical modification of enzyme molecules [2]. As an effective modification method, limited hydrolysis can usually result in some beneficial change for an enzyme in chain and conformation and thus can alter the characteristics and functions of the enzyme. For examples, trypsinogen does not exhibit catalytic activity before a six-peptide is removed from the molecule via protease hydrolysis [3] and the activity of asparaginase can be increased four to five folds after its 10 or more amino acid residues are removed from its carboxyl terminal through trypsin hydrolysis [4,5].
As a hydrolases, lipase (triacylglycerol ester hydrolases, EC 3.1.1.3) catalyzes the hydrolysis of triglyceride to free fatty acids, diacylglycerol, monoglyceride and glycerol. Lipase also shows efficiency in various reactions such as ester synthesis, transesterification, interesterification, acidolysis and aminolysis in organic solvent or on support materials [6,7], depending on the reaction conditions. Therefore, lipase is currently widely used in food industry, chemical, oleochemicals, agro-chemical, paper making, washing, and synthesis of biosurfactants, biodiesel production and other industries [8,9]. Recently, some researchers have been carried out to improve lipase activity for extended applications, such as enzymatic hydrolysis, chemical modification, gene recombination and immobilization and so on [6,10,11]. Limited hydrolysis of enzyme via the role of protease belongs to the method of enzyme modification, through which the activity and other properties of enzyme, such as the affinity toward substrates, the optimum reaction condition and heat-reliability, may get beneficially changed due to the modification of molecular structure. Consequently, the application of enzymes is got extended, such as the production of functional esters with modified lipase. The aim of this paper is to find out the effect of trypsin hydrolysis on the activity of lipase. Also the change of the trypsin-treated lipase, including the enzymatic properties and thermal stability are characterized.
Materials
Lipase Palatase 20000L was purchased from Novozymes Company 584±13U mL-1, from Aspergillus oryzae, Novozymes Switzerland AG, Dittengen, Switzerland, and trypsin was purchased from Sigma- Aldrich Company (2500±30U mg-1, from porcine pancreas, Sigma Chemical Co., Louis, USA).
Activity assay of lipase
One unit of lipase activity (U) was defined as the enzyme amount required liberating 1μmol fatty acid per minute under the following assay conditions. Lipase activity assay was performed according to the olive oil emulsion method reported by Pawinee & Suree [12]. The olive oil substrate was emulsified at 1:3 ratio with distilled water containing 2% (w/v) polyvinyl alcohol in an ultrasonic sonicator. An ultrasonic homogenizer (model JY92-iiDN from Ningbo Scientz Biotechnology Co. LTD. Ningbo city, China) was used to generate an ultrasonic wave of 20kHz at 500W and a tapered microtip probe (6mm in diameter) was immersed into the sample solution at a 1.0-1.5cm distance from the liquid level. A 3-3 second pulse-on mode and 6 min of total processing time were set. A reaction mixture was prepared with 4mL olive oil emulsion substrate, 4mL phosphate buffer (pH7.5, 0.05mol L-1) and 50μL lipase pre-heated at 37°C respectively and the hydrolysis was carried out at 37°C for 20 min in a water bath shaker at 180 r min-1. Hydrolysis reaction was terminated by adding 15mL ethanol (95%) to the reaction mixture. The liberated fatty acids were titrated against NaOH standard solution (0.05mol L-1) with phenolphthalein as the indicator. The control experiments were performed under the same conditions with phosphate buffer substitute for enzyme.
Treatment of lipase with trypsin
50μL of the lipase was incubated with 1ml trypsin solution at different concentrations (0.5-4mg mL-1 , dissolved in 0.001mol L-1HCl) and 4mL of buffer (0.05mol L-1, pH5.0-9.0) in a serial conical flasks at various temperatures (25°C-50°C). After incubation for the scheduled time (0-60min), the activity of the treated lipase was determined at 37°C according to the method as described above. An equal volume of 0.001mol L-1 HCl was used as the blank. All experiments were carried out in triplicate.
To distinguish the hydrolysis effect of the trypsin on lipase from the hydrolysis effect of trypsin on the emulsified olive oil substrate hydrolysis, the same olive oil emulsion was used as substrate to incubate with the trypsin solution at pH7.5 and 37°C for 20 min in a water bath shaker at 180 rpm. After reaction, 15mL ethanol (95%) was added to the mixture to terminate trypsin activity and the liberated fatty acids were then titrated. All experiments were carried out in triplicate.
Determination of Kinetic parameters
The kinetic parameters of the trypsin-treated lipase and native lipase were determined according to the Lineweaver-Burk method. The kinetic constant value (Km) was determined according to the initial reaction rates of the trypsin-treated and native lipase at various concentrations of the emulsified olive oil substrate in phosphate buffer (0.05 mol L-1, pH 7.0) at 37°C within 20 minutes. All experiments were carried out in triplicate. Lineweaver-Burk plots were constructed by plotting the reciprocal of the enzyme reaction velocity (1 v-1) versus the reciprocal of the substrate concentration (1 [S] -1). The Michaelis constant (Km) and maximum reaction velocity (Vmax) were obtained from the negative reciprocal of the intercept with the 1 s-1 axis and the reciprocal of the intercept with the 1 v-1 axis, respectively.
Comparation of thermal stability
Change of the trypsin-treated lipase and native lipase in thermal stability was compared at 40°C, 45°C, 50°C and 60°C by determining the activity during the incubation from 1 to 90 minutes respectively. Thermal deactivation kinetics of the trypsin-treated lipase and native lipase was compared based on a single step first-order deactivation model and sequential deactivation model involving one and two intermediate states according to Sá-Pereira et al. [13]. The three-step deactivation model is expressed as
K1 α1 K2 α2
E→ E1 → E2
Where E, E1 and E2 are the different enzyme states; k1 and k2 are the first-order deactivation rate coefficients; α1 and α2 are the ratio of the specific activity of E1 and E2 to the specific activity of E, respectively. According to Sá-Pereira et al. [13], two-step deactivation model can be adopted to simulate most of the enzyme thermal deactivation. In the case that α1<1, the intermediate state has a specific activity lower than the initial enzyme state; in the case that α1=1, the intermediate state is as active as the initial enzyme state; and in the case that α1>1, the intermediate state has a higher specific activity than the initial enzyme state. The similar consideration can be assumed for α2. If the contents of E1 and E2 are equal to zero at the beginning, the residual activity (a) at time t can be expressed by
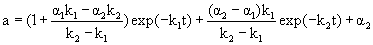 (1)
(1)
In the case that α2=0 and the enzyme state E2 is completely deactivated and equal to the final enzyme state, the deactivation of enzyme conforms to the two-step deactivation model. The residual activity (a) can be expressed by
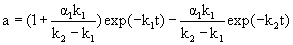 (2)
(2)
In the case that α1=0 and E1 is the final state of the enzyme, the deactivation of enzyme conforms to the single step first-order deactivation model. The residual activity (a) can be expressed by
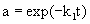 (3)
(3)
Effect of trypsin on the activity of lipase
Lipase was found to be increased in activity from initial 584±13U mL-1 to maximum 759±15U mL-1 after treatment of trypsin at 1.5mg mL-1 concentration at 30°C and pH7.0 for 30min (Figure 1a). Such effect of trypsin on lipase activity is likely contributed to the results from the limited hydrolysis by lipase or the olive oil hydrolysis directly due to trypsin activity. To distinguish these two kinds of effect, trypsin solution was separately incubated with the same olive oil emulsion substrate at pH7.5 and 37°C for 20 min in a water bath shaker at 180 rpm. After the scheduled reaction time, 15mL ethanol (95%) was added to the mixture to eliminate trypsin activity and the liberated fatty acids were titrated. The result showed that no liberated fatty acid was detected, indicating that trypsin shows no direct hydrolysis effect on olive oil substrate. The increased liberated fatty acid content in the reaction mixture after reaction is only resulted from the improved lipase activity. Trypsin had been also found to have activation effect on aspartase [4]. Keiko & Masanobu found that the activation of aspartase from Escherichia coli by trypsin required a few minutes to attain a maximal level, and hereafter the enzyme activity gradually decreased resulting in a complete inactivation in about 4 hours. Prior or intermediate addition of soybean trypsin inhibitor resulted in an immediate cessation of any further change in the enzyme activity, indicating the role of the limited hydrolysis of trypsin. Other researches also reported the activation effect of trypsin on some kinds of enzymes by limited hydrolysis. Shanthy et al. [14] found that trypsin-treated P-glycoprotein ATPase exhibited change in function. Trypsin-treated porcine procolipase, procollagenase, procarboxypeptidase B and human matris metalloproteinase-9 were also found to be activated [15-18]. Such an activation of these enzymes is very complicated and may be linked to the change of proteins in peptide chain and higher conformations, therein the active centre of the enzymes is affected substantially and rationally [19]. In other words, beneficial change of enzyme peptide chain is first resulted from the appropriate limited hydrolysis and then the conformation change and improved activity change may be the consequent results.
Lipase activity based on treatment conditions by trypsin
The treatment conditions, involving the trypsin concentration (activity), treatment temperature, treatment time and pH value mainly affect the activity of lipase via impact on the limited hydrolysis degree of lipase. Hence the effect of these factors on lipase activity was studied. The effect of trypsin concentration on lipase activity was carried out by incubating the lipase solution with 1mL of trypsin solution from 0.5mg mL-1 to 4mg mL-1 at 30°C and pH 7.0 for 30min. The maximum of the lipase activity reached about 759U mL-1. It was found that at the trypsin concentrations lower than 1.5mg mL-1, lipase activity increased as trypsin concentration was increased (Figure 1a), while as the trypsin concentrations were increased higher than1.5mg mL-1, lipase activity was reduced markedly, indicating that excessive hydrolysis by trypsin can destroy lipase activity. The effect of treatment temperature on lipase activity was carried out by incubating trypsin with lipase solutions (50μL of lipase, 4mL of pH7.0 PBS and 200μL of trypsin, at 5mg mL-1) at 25°C, 30°C, 37°C, 45°C and 50°C for 30min respectively. Figure 1b shows the change of the lipase activity after trypsin treatment. The maximum activity of lipase appeared at 30°C (about 713U mL-1). Lipase activity was found to be decreased obviously at the temperatures higher than 37°C. The effect of treatment time on lipase activity was carried out by incubating the trypsin with lipase solutions (50μL of lipase, 4mL of pH7.0 PBS and 200μL of trypsin, at 5mg mL-1) at 30°C for 0-60min respectively. The result is shown as Figure 1c. The maximum lipase activity appeared at the 30th min of the trypsin treatment (about 720U mL-1). Extended treatment time showed a feeble increase in lipase activity. The effect of treatment pH value on lipase activity was also carried out by incubating the trypsin with lipase solutions (50μL of lipase, 1mL of PBS from pH5-9 and 200μL of trypsin, at 5mg mL-1) at 30°C for 30min respectively. The result is shown as Figure 1d. It was found that lipase activity was affected markedly by pH value of the treatments. The maximum lipase activity appeared at pH7.0-8.0 (about 738U mL-1). At the pH values higher than 8.0, lipase activity was observed to be reduced sharply and even lower than the activity of the native lipase (584U mL-1) at pH9.0
Activity profiles of the trypsin-treated lipase at various pH values and temperatures
The activity profiles of the trypsin-treated lipase at various pH values were assayed and compared with the native lipase by determining their activities in the buffers from pH4-10, at 37°C, shown as Figure 2. The activity profiles of the trypsin-treated lipase at various temperatures were also assayed and compared with the native lipase by determining their activities at the temperatures from 25°C to 55°C at pH 7.0, shown as Figure 3. Results showed that the optimum pH value of the trypsintreated lipase was at around 8.0, kept basically unchanged. This result is similar to the findings of Lee et al on the trypsin-treated aspartase, in which aspartase from Hafnia alvei was found to be activated by trypsin and the optimum pH of the trypsin-treated aspartase was also essentially unchanged [20]. The optimum temperature for the trypsintreated lipase was detected at around 40°C, lower than the native lipase (50°C), suggesting the much higher heat sensitivity of the trypsintreated lipase. The bell shape of the curves results from increasing rate of reaction at the lower temperatures and declining enzyme activity due to denaturation at higher temperatures.
Kinetic properties
Figure 4 shows the Lineweaver-Burk plots for the trypsin-treated lipase and native lipase. The Michaelis constant (Km), which corresponds to substrate concentration that gives one-half of the maximum reaction velocity, was calculated from the negative reciprocal of the intercept with the 1/s axis. The Km values for the trypsin-treated lipase and native lipase were calculated as around 79mg mL-1 and 100mg mL-1 respectively. The Vmax values for the trypsin-treated lipase and native lipase were calculated as around 56.18 and 49.75μmol fatty acid min-1 respectively. The Km values signify the extent to which the enzymes have access to the substrates [21]. Smaller the Km values, higher the affinity of enzyme toward substrates. So the lower Km value for trypsintreated lipase indicates that hydrolysis by trypsin increases the affinity of the enzyme for olive oil substrate. Compared to the native lipase, the activity and affinity of enzyme toward substrates of trypsintreated lipase were found to be improved. This improvement would be beneficial for increase of substrate conversion rate and reduction of reaction time and reaction cost. The heat-reliability and reusability of trypsin-treated lipase may be improved by further modification such as immobilization and chemical modification.
Thermal stability
The thermal stability of the trypsin-treated lipase and native lipase at 40°C, 45°C, 50°C and 60°C were studied and compared respectively, shown as Figure 5 and Figure 6. It was found that the activity of both trypsin-treated lipase and native lipase at 45°C maintained unchanged basically within the almost whole period of incubation (90min). However, the decreased tendency was observed at the temperatures higher than 45°C for the trypsin-treated lipase and higher than 50°C for the native lipase. For both the trypsin-treated and native lipases, decrease in stability was found to be as the function of time. Hence the sequential deactivation based on three-step and two-step models was proposed for the deactivation of the trypsin-treated lipase at 50°C and 60°C respectively. These suggested three-step model and two-step model, represented as Equation (1) and Equation (2), were found to be correlated well with the experimental data (Figure 5). While at 45°C, the data was found to fit well with a single-step (first-order) deactivation mechanism (Equation (3)). The half-inactivation time for the trypsin-treated lipase at 45°C, 50°C and 60°C were calculated as 131min, 35.5min and 4min respectively. The deactivation parameters of the trypsin-treated lipase, such as α1, α2, k1, k2 and t1/2 values are showed as Table 1. Because the activity of trypsin-treated lipase was nearly unchanged during the whole 90min at 40°C, so the deactivation parameters at 40°C are not showed in the Table 1. For the native lipase, the experimental deactivation dates exhibited as the function of time at 50°C and 60°C and were adjusted based on a single step and three-step respectively (Figure 6). From Figure 6 the predicted data are found to be correlated well with the experimental data. The halfinactivation time for the native lipase at 50°C and 60°C were calculated as 128min and 13min respectively. The deactivation parameters of the native lipase, such as α1, α2, k1, k2 and t1/2 values are showed in Table 1. Similarly, the activity of the native lipase was nearly unchanged during the whole 90min at 45°C, so the deactivation parameters at 45°C were not showed in the Table 1. These results suggestion consequently that the thermal stability of the trypsin-treated lipase is lower than the native lipase. Namely, limited hydrolysis of lipase by trypsin results in higher sensitivity to heat.
| trypsin-treated lipase | trypsin-treated lipase | native lipase | trypsin-treated lipase | native lipase | |
|---|---|---|---|---|---|
| K1 | 0.00528 | 0.18 | 0.0054 | 0.31 | 0.37 |
| K2 | n.a.** | 0.031 | n.a. | 0.048 | 0.034 |
| α1 | n.a. | 0.73 | n.a. | 0.322 | 0.71 |
| α2 | n.a. | 0.34 | n.a. | n.a. | n.a. |
| t1/2(min) | 131 | 35.5 | 128 | 4 | 13 |
* The deactivation parameters of trypsin-treated lipase are calculated based on the kinetic deactivation schemes (the first-order, three-step and two-step kinetic mechanism corresponding to Eq. 3, Eq. 1 and Eq. 2 respectively). The deactivation parameters of the native lipase are calculated based on the kinetic deactivation schemes (the firstorder and two-step kinetic mechanism corresponding to Eq. 3 and Eq. 2 respectively).
**n.a.: not applicable.
Table 1: Deactivation parameters of trypsin-treated lipase at 45, 50 and 60°C and native lipase at 50 and 60°C.
The activity, reaction kinetics and thermal-reliability of lipase are affected by trypsin hydrolysis. Thereinto, the activity of the trypsintreated lipase is improved and the Km value is lower than the native lipase. The optimum temperature and thermal stability of trypsintreated lipase are lower than the native lipase.
The authors thank the Ministry of Science and Technology of the People’s Republic of China (2010AA101505) for the financial support.