Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2011) Volume 2, Issue 1
This study was designed to determine the structural changes of human serum albumin, HAS, in the presence of aspirin in H 2 O solutions at physiological pH 7. Isothermal titration calorimetry, ITC, at 298 and 310K, and curve-fitting procedures were applied to characterize the drug binding sites, the binding constants in the aspirin+HSA complexes. The heats obtained for aspirin+HSA interactions are reported and analyzed in terms of the extended solvation theory. It was indicated that there are a set of two identical and non-cooperative sites for aspirin. Calorimetric evidence showed that strong aspirin+HSA interaction occurs at very low aspirin concentration (0.007-0.084 mM), with overall binding constants of 2.76 × 10 6 M -1 and 9.3×10 5 M -1 at low and high aspirin concentration domains respectively.
Keywords: Huamn serum albumin; Isothermal titration calorimetry; Aspirin; Binding parameters
The energy of biochemical reactions or molecular interactions at constant temperature is measured by isothermal titration calorimetry. At present, ITC is used to study all type of binding reactions, including protein-protein, protein-ligand, DNA-drug, DNA-protein and enzyme kinetic [1-5]. HSA is the most abundant protein in systemic circulation, with HSA comprising 60% in plasma. Serum proteins have many important functions that are of great interest in pharmaceutical science, medical science, biology, and chemistry; some of them are related to the fixation and transport of metabolites, hormones, and exogenous substances like pharmaceutical drugs [6-9]. It is a relatively small (65 kDa), highly soluble protein consisting of 585 amino acids, with 17 disulphide bridges, on free thiol group (Cys-34) and a single tryptophan (Trp-214) [10]. Albumin consist of three homologous domains and each of them is divided into two subdomains, A and B. Binding sites that are characteristic for most of the drugs are located in subdomains ΙΙA and ΙΙΙA. HSA is a widely studied protein because its primary structure is well known and recently, the three-dimensional structure of HSA was determined through X-ray crystallographic measurements [11,12]. The unique feature of albumin is its ability to bind a wide variety of compounds, mainly because of the availability of hydrophobic pockets inside the protein network and the flexibility of the albumins to adapt their shape [13,14]. HSA is known to possess several set of binding sites for different classes of drugs [15-17]. Spectroscopic evidences showed that no aspirin-protein interaction occurs at very low aspirin concentration (0.0001 mM), whereas at higher drug contents (0.001-0.1 mM) the aspirin anion binding with HSA (H-bonding) is mainly through the amino group with overall binding constant of K=1.4 × 104 M-1. Aspirin binding results in protein secondary structural changes from that of the α-helix 55% (free HSA) to 49%, β-sheet 22% (free HSA) to 31%, β-anti 12% (free HSA) to 4% and turn 11% (free HSA) to 16% in the aspirin+HSA complexes [18]. The affinity constants of the albumin binding sites for small pharmaceutical drugs have usually been determined using equilibrium dialysis, a time-consuming technique, because the low values of association constants (Ka = 106 M-1) hamper the separation of the bound from the free ligand using physical methods that involve filtration or centrifugation procedures. In this work, we have attempted to find the binding parameters and conformational changes of HSA due to its binding with aspirin at pH 7.
The isothermal titration calorimetric experiments were carried out on a VP-ITC ultra sensitive titration calorimeter (MicroCal, LLC, Northampton, MA). The microcalorimeter consists of a reference cell and a sample cell of 1.8 mL in volume, with both cells insulated by an adiabatic shield. All solutions were thoroughly degassed before use by stirring under vacuum. The sample cell was loaded with aspirin solution (0.5 mM) and the reference cell contained buffer solution. The solution in the cell was stirred at 307 rpm by the syringe (equipped with micro propeller) filled with aspirin solution (30mM) to ensure rapid mixing. Injections were started after baseline stability had been achieved. The titration of HSA with aspirin solution involved 30 consecutive injections, the first injection was 5 μL and the remaining ones were 10 μL. In all cases, each injection was done in 6s at 3-min intervals. To correct the thermal effects due to aspirin dilution, control experiments were done in which identical aliquots were injected into the buffer solution with the exception of HSA. In the ITC experiments, the heat changes associated with processes occurring at a constant temperature are measured. The measurements were performed at a constant temperature of 27.0±0.02°C and the temperature was controlled using a Poly-Science water bath. The determined heats for aspirin+HSA interaction were listed in Table 1 and shown graphically in Figures 1 and 2. The microcalorimeter was frequently calibrated electrically during the course of the study.
| [ASP]/µM | q / µ J (o) | qdilut /µ J (o) | q / µ J (∆) | qdilut /µ J (∆) |
| 7.0 | -125.3 | -18.4 | -94.9 | -18.4 |
| 10.5 | -125.5 | -15.6 | -95.7 | -15.6 |
| 13.9 | -125.8 | -13.5 | -95.9 | -13.5 |
| 17.2 | -125.8 | -11.5 | -96.7 | -11.5 |
| 20.5 | -125.4 | -10.3 | -95.5 | -10.3 |
| 23.8 | -123.9 | -9.4 | -93.2 | -9.4 |
| 27.0 | -120.5 | -6.9 | -85.8 | -6.9 |
| 30.2 | -113.2 | -6.1 | -76.5 | -6.1 |
| 33.3 | -108.0 | -4.9 | -74.1 | -4.9 |
| 36.4 | -105.0 | -4.2 | -74.7 | -4.2 |
| 39.4 | -104.8 | -2.7 | -77.0 | -2.7 |
| 42.5 | -105.1 | -1.9 | -79.2 | -1.9 |
| 45.4 | -104.8 | -0.6 | -80.7 | -0.6 |
| 48.4 | -104.9 | 0.3 | -82.2 | 0.3 |
| 51.2 | -105.2 | 1.7 | -83.2 | 1.7 |
| 54.1 | -105.4 | 2.6 | -83.9 | 2.6 |
| 56.9 | -105.7 | 3.3 | -83.8 | 3.3 |
| 59.7 | -105.1 | 3.4 | -84.0 | 3.4 |
| 62.5 | -103.0 | 3.4 | -83.3 | 3.4 |
| 65.2 | -101.4 | 2.9 | -82.0 | 2.9 |
| 67.9 | -100.8 | 4.3 | -82.2 | 4.3 |
| 70.5 | -98.9 | 4.1 | -81.3 | 4.1 |
| 73.1 | -97.2 | 3.2 | -79.9 | 3.2 |
| 75.7 | -95.5 | 2.9 | -78.9 | 2.9 |
| 78.3 | -94.8 | 3.9 | -78.8 | 3.9 |
| 80.8 | -92.0 | 3.5 | -78.1 | 3.5 |
| 83.3 | -87.7 | 1.9 | -74.2 | 1.9 |
Table1: Heats of aspirin+HSA interactions, q, at 298 K (o) and 310 K (Δ) in H2O solution of pH=7. qdilut are the heats of dilution of aspirin with water. The precision is ±0.1 μJ or better.
We have shown previously [19-23] that the heats of the macromolecules+ligands interactions in the aqueous solvent systems can be reproduced by the following equation:
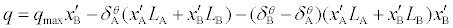 (1)
(1)
The parameters 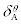 and
and 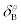 reflect to the net effect of aspirin on the HSA stability in the low and high aspirin concentrations respectively. The positive values for
reflect to the net effect of aspirin on the HSA stability in the low and high aspirin concentrations respectively. The positive values for 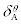 or
or 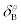 indicate that aspirin stabilizes the HSA structure and vice versa.
indicate that aspirin stabilizes the HSA structure and vice versa. 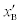 can be expressed as follows:
can be expressed as follows:
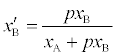 (2)
(2)
p > 1 or pis the fraction of bound and 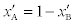 is the fraction of unbound aspirin. We can express xBas follows:
is the fraction of unbound aspirin. We can express xBas follows:
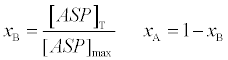 (3)
(3)
[ASP]T isthe total concentration of aspirin and [ASP]max is the maximum consternation of aspirin upon saturation of all HSA. LA and LB can be calculated from heats of dilution of aspirin in buffer solution, qdilut , as follows:
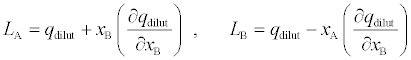 (4)
(4)
The heats of aspirin+HSA interactions were fitted to Eq. 1 over the whole aspirin concentrations. In the fitting procedure, the only adjustable parameter (p) was changed until the best agreement between the experimental and calculated data was approached. The small relative standard coefficient errors and the high r2 values (0.9999) support the method. The binding parameters for aspirin+HSA interactions recovered from Eq. 1 were listed in Tables 2 and 3. 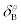 values for aspirin+HSA interaction are positive, indicating that in the high concentrations of aspirin, HSA structure is stabilized (specific interactions).
values for aspirin+HSA interaction are positive, indicating that in the high concentrations of aspirin, HSA structure is stabilized (specific interactions). 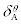 values are negative, implying that HSA is destabilized in the low concentration of aspirin, indicating that the non-specific interactions (i.e. N- and O-containing groups) have no contribution in HSA stabilization in the low aspirin concentration. Aspirin interacts with HSA at two distinctive set of binding sites that are clear in (Figure 1). The association equilibrium constants for the first set of binding sites with higher affinity are 2.76×106 M-1, while the second set of sites has a much lower affinity with Ka= 9.3×105 M-1. The first set of binding sites begin to be occupied by aspirin and influences the total heat, in a greater proportion because aspirin bound to the first set of binding sites has an affinity 3 times higher than aspirin bound to the second set of binding sites.
values are negative, implying that HSA is destabilized in the low concentration of aspirin, indicating that the non-specific interactions (i.e. N- and O-containing groups) have no contribution in HSA stabilization in the low aspirin concentration. Aspirin interacts with HSA at two distinctive set of binding sites that are clear in (Figure 1). The association equilibrium constants for the first set of binding sites with higher affinity are 2.76×106 M-1, while the second set of sites has a much lower affinity with Ka= 9.3×105 M-1. The first set of binding sites begin to be occupied by aspirin and influences the total heat, in a greater proportion because aspirin bound to the first set of binding sites has an affinity 3 times higher than aspirin bound to the second set of binding sites.
| parameters | First binding sites | Second binding sites |
| p | 1 | 1 |
| gi | 6.00±0.18 | 8.00±0.12 |
| Ka / M-1 | 2760993.60±49.00 | 928352±16.18 |
| ΔH/ kJ mol-1 | -19.90±0.04 | -18.38±0.03 |
| ΔG/ kJ mol-1 | -36.71±0.09 | -34.01±0.11 |
| ΔS / kJ mol-1K-1 | 0.056±0.002 | 0.052±0.002 |
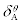 |
-0.31±0.03 | |
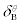 |
1.07±0.09 |
Table 2: Binding parameters for ASP+HSA interaction recovered from Eqs.1 and 2 at pH 7 and 298K. p=1 indicates that the binding is non-cooperative in the two sets of binding sites. The positive value of 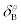 indicate that ASP+HSA complex is stable and the negative value of
indicate that ASP+HSA complex is stable and the negative value of 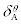 imply that aspirin destabilizes the HSA structure due to its interaction with –CO and –NH2 groups of amino acids (non-specific interactions) in the low aspirin concentration region. The interaction of aspirin with HSA is strong as indicated by big equilibrium association constants.
imply that aspirin destabilizes the HSA structure due to its interaction with –CO and –NH2 groups of amino acids (non-specific interactions) in the low aspirin concentration region. The interaction of aspirin with HSA is strong as indicated by big equilibrium association constants.
We have introduced an empirical equation which is suitable for fitting of this complicated system including two sets of binding sites on HSA, as follows:
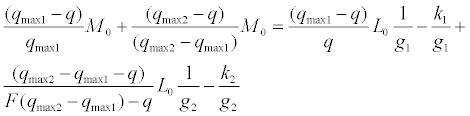 (5)
(5)
where F parameter can be define as follows:
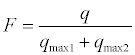
A non-linear least squares computer program has been developed to fit data in Eq. 2. The best correlation coefficient (R2 ≈ 1) and the least standard deviations (SD ≈ 0.01or better) are good support for the use of Eq. 2. The binding parameters recovered from Eq. 2 (K1, K2, g1 and g2) were listed in Tables 2 and 3.
| parameters | First binding sites | Second binding sites |
| p | 1 | 1 |
| gi | 6.00±0.21 | 8.00±0.27 |
| Ka / M-1 | 547198.10±38.00 | 482163.14±22.18 |
| ΔH/ kJ mol-1 | -10.57±0.08 | -13.30±0.06 |
| ΔG/ kJ mol-1 | -34.02±0.11 | -33.70±0.14 |
| ΔS / kJ mol-1K-1 | 0.056±0.002 | 0.066±0.003 |
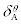 |
-0.27±0.08 | |
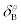 |
2.72±0.12 |
Table 3: Binding parameters for ASP+HSA interaction recovered from Eqs.1 and 2 at pH 7 and 310K. p=1 indicates that the binding is non-cooperative in the two sets of binding sites. The positive value of 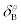 indicate that ASP+HSA complex is stable and the negative value of
indicate that ASP+HSA complex is stable and the negative value of 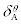 imply that aspirin destabilizes the HSA structure in the low aspirin concentration region. The interaction of aspirin with HSA at 310K is weaker than that of at 298K as indicated by smaller equilibrium association constants.
imply that aspirin destabilizes the HSA structure in the low aspirin concentration region. The interaction of aspirin with HSA at 310K is weaker than that of at 298K as indicated by smaller equilibrium association constants.