Journal of Applied Pharmacy
Open Access
ISSN: 1920-4159
ISSN: 1920-4159
Research Article - (2020)Volume 12, Issue 1
The chick Chorioallantoic Membrane (CAM) is a possibly three-dimensional representation that can be used for in vivo as well as in situ studies. Its relatively easily available, relatively less expensive and consistent in quality render it an appropriate biological model for the use in experiments requiring live tissues. The aim of current research was to determine the angiogenic/antiangiogenic effect of Aceclofenac Sodium (AcS) and the effective dose of AcS. Total 30 fertilized chicken eggs (5 days old) were obtained from a Big bird Pvt. Ltd (local hatchery). Five groups were formed. They were incubated at 37°C with humidity of 55-60%. An opening about 2 cm of diameter was created under aseptic conditions by removing the shell and inner shell membrane. On 6thday of fertilization, Group A was given 0.1 ml Phosphate Buffer Saline (PBS) and served as control, group B, C, D and E were given 0.64 mg/0.1 ml, 0.32 mg/0.1 ml, 0.16 mg/0.1 ml, and 0.08 mg/0.1 ml of AcS respectively. Eggs were then again sealed with paraffin film under sterile environment and were kept in incubator for next 24 hours. After incubation period, the CAM was exposed and pictures were taken. The growth of blood vessels, diameter, branching pattern, surface roughness and other characteristics were evaluated by using Adobe Photoshop and Scan Probing Image Processing (SPIP). AsS showed dose dependent dual effect. At low concentration (0.08 mg/ml) it showed anti-angiogenic effect whereas at higher concentration (0.64 mg/ml) it produced angiogenic results. This indicates that AsS can be beneficial for development of strategies for prevention and therapy for several types of angiogenesis dependent diseases.
Aceclofenac Sodium (AcS); Chorioallantoic Membrane Assay (CAM); Angiogenesis
New blood vessels developing from previous capillaries termed as angiogenesis [1]. This phrase was used in 1935 to define production of new blood vessels in placenta [2]. Owing to the involvement and supply of nutrients in the blood through a vascular network as well survival of cell only depends on angiogenesis. Therefore, angiogenesis is extremely significant to number of functions in physiology for example wound healing, menstruation, developing embryo and normal tissue growth [1]. Similarly, the incompetence of the body to accurately control this phenomenon of angiogenesis can lead to many severe diseases such as ischemic tissues and cardiac failure. In the same way, abnormal high level of angiogenesis can lead to various pathological conditions like cancer, cardiac problems (arthrosclerosis), severe soreness (rheumatoid arthritis, Crohn's Disorder), age-related macular degeneration ADM, damage of retina due to diabetes mellitus, endometriosis, psoriasis, and adiposity [3,4]. Endothelial cells lines that are in straight interaction with blood stream. Underneath this single layer of epithelial cells, blood vessels are surrounded by pericytes, adventitial cells, formative cells, fine textured muscle cells, extracellular and basement membranes [1]. These metabolically active epithelial cells perform an important role in several mechanisms in the body. These include regulation of blood coagulation guiding the immune cells to particular areas in the body by releasing of chemo archetectonic, chemokines and cytokines, assisting in vascular altering and sprightly being comprised in the angiogenic cascade [1,5,6]. This angiogenic process is initiated by pro-angiogenic factor release that activates signalling cascade. The influence releasing of pro angiogenic parameter is generally a response of delivery of various protein through the immune system in an ischemic environment [1]. The transformation from inactive vasculature to angiogenic vasculature is mark able modifications in the equilibrium between pro- and anti-angiogenic factor. Chorioallantoic Membrane (CAM) model can be used in the investigation for anti-angiogenic drugs single as well in group. In current decade, CAM assay has become very popular and is now frequently used in the study of angiogenesis [7]. This model delivers a usual, in vivo setting of angiogenic blood vessels with all the multifaceted swarm communication on which angiogenic combination can be verified [7]. The key benefit of this exemplary is its direct contact to cell, tissues, biomaterial, drugs or other methods of treatment in order to control their special effects on anfiogenesis [7]. CAM is a peritoneum that surrounds the foetus by the blending of extra-embryonic membrane, the chorion and allantois, on 4 day of Embryonic Developed (EDD) [7]. It delivers some rudimentary physical and biological functions desired for the embryo to survive including, respiratory functions, absorbing calcium from eggshell, providing a pool for waste products of embryo (initially urea then mostly uric acid), and absorption of albumin by the allantois [7]. In order to perform these functions, the CAM develops as a highly vascularized membrane in direct contact with the eggshell. There are different alternative models exist, that are used to investigate angiogenesis process, both in vivo and in vitro. Angiogenesis is involved in the disruption of basement membrane, cell migration, cell burst as well tube development, and all of these studies are individually performed in vitro. In vitro models can be provide a lot of information, but they are limited in their ability to stimulate more complicated pathways and interactions involved in biological processes, creating a need for in vivo models as well [7]. CAM model has number of variation two are as follows, 1-ex vivo, 2-in vivo models. This research work was completed by the in ovo model. In the in ovo model, the eggs is left to develop inside of the eggshell and the CAM is accessed by creating a hole in the eggshell, referred to as windowing, and allowing the membrane to drop and develop detached from the eggshell in a certain area [7]. This strategy gives the favourable position that it keeps up a more physical and biological settings for progression of the stratum, in any case, it is restricted because of the way that lone a little part of the CAM is open, and there is an expanded shot that the membrane may not drop appropriately, constraining access to the CAM at the time or in the way fancied [7]. Pictures of the CAMs vasculature were made by using DSL camera. These pictures were treated by two separate commands recorded in SPIP version. One of the programs analysed the inhibition of developing CAM due to anti-angiogenic activity of a drug and quantification of redevelopment of blood vessels. AcS is a Non-Steroidal Anti- Inflammatory Drug (NSAID) equivalent of diclofenac. AcS is indicated as anti-pyretic and anti-inflammatory for rheumatoid arthritis osteoarthritis and Bekhterev's disease. It is specified to have a great action against inflammation and show relative impact than other NSAIDs in double-blind study. Aceclofenac potently inhibit the Cyclooxygenase Enzyme (COX) which is involved in the production of prostaglandins, which are inflammatory mediators that cause swelling, pain, fever and inflammation. In this research, the novelty is that AcS can be used in inhibition of cancer, it will be new use of NSAID, along with anti-inflammatory property, it has anti-cancerous potential. Without angiogenesis, the tumour cannot increase its size above the size of head of common pin. Cancer develop only after angiogenesis as the new blood vessels supply the nutrients and help in its spread, give stress on its explanation for use in cancer.
• To assess the angiogenic/anti-angiogenic effect of AcS.
• To assess the effective concentration of AcS for angiogenesis.
Chemicals and materials
This research work was done for the evaluation of angiogenic and anti-angiogenic property of AcS. AcS was purchased from sigma Aldrich. Blood vessels were recorded through DSL Camera. A 0.9% normal saline solution, which was bought from Searle Pakistan Pvt Limited, is used as a medium for dilutions of drug. Fertilized eggs were bought from local hatchery Big Bird Pvt Ltd. Injections into the CAMs vasculature were made through Microliter Syringes fortified with 33-G metal needles. Fertilized eggs (5 days old) of chicken were obtained from a local hatchery Big Bird Poultry Pvt Limited. They were incubated at 37°C with humidity of 55-60%. Then, on fifth day of incubation, small window of about 2 cm of diameter were created by removing the shell and then internal shell membrane. It was done under aseptic conditions. Five groups were formed. Group A was given 0.1 ml Phosphate Buffer Saline (PBS) and kept as control, group B, C, D and E were given 0.64 mg/0.1 ml, 0.32 mg/0.1 ml, 0.16 mg/0.1 ml and 0.08 mg/0.1 ml respectively. Then pH of all solutions was checked with pH meter and was adjusted to 6-7.4. In order to reduce chance of contamination all the prepared solutions were filter by 0.2 μ syringe filters. On 6th day, the prepared dilutions of AcS were injected and eggs were again affixed with paraffin film in sterile setting. These were put back in incubator for following 24 hours. After twenty four hours, eggs were taken out from incubator and picture of all eggs i.e., control as well as those contracts with all doses of AcS were produced by using a DSL camera. The growth of blood vessels and other characteristics were evaluated by used Adobe Photoshop version 7.0, and then these pictures were shift to Scan Probing Image Processing (SPIP) software 6.6.2. The diameters of primary, secondary and tertiary blood vessels were measured. The framework described the extent of inhibition of naturally developing CAM includes, the diameter and branching system of blood vessels measured as per mm, and categories of blood vessels, i.e., primary, secondary and tertiary blood vessels. Individual x, y and z measurements of every picture were stacking for decided diverse parameters to evaluate angiogenesis. The widths of different blood vessels were measured by utilizing adjustment and estimation command.
Image-processing quantification method
Quantification of images was performed on images taken from the DSL cameras used a macro specifically for SPIP and existing plugins., the image fulfilled the criteria as follows, a recorded resolution at 1280ᵡ 1024 dynamic range of 12 bits and 4095 grey levels, magnification with ᵡ10 objectives and high contrast obtained. To describe the grade of inhibition of the naturally developing CAM following parameters including, the diameter and branching system of blood vessels measured as per mm, and categories of blood vessels i.e., primary, secondary and tertiary blood vessels were done.
Statistical analysis
Data was assessed on SPSS statistical software version 22.0 using one way ANOVA.
Qualitative analysis
AcS is investigated for the inhibition of physiologically growing CAM membrane in this study. The treatment of CAM with anti-angiogenic compound during the phase of development characterized by exponential vascular growth prevents the regular growth of capillaries and vessels that indicate anti angiogenic property of AcS. The result of this drug is shown in Figure 1. It shows that AcS is angiogenic at higher concentration (0.64 mg/0.1 ml) and anti-angiogenic at low concentration (0.08 mg/0.1 ml).
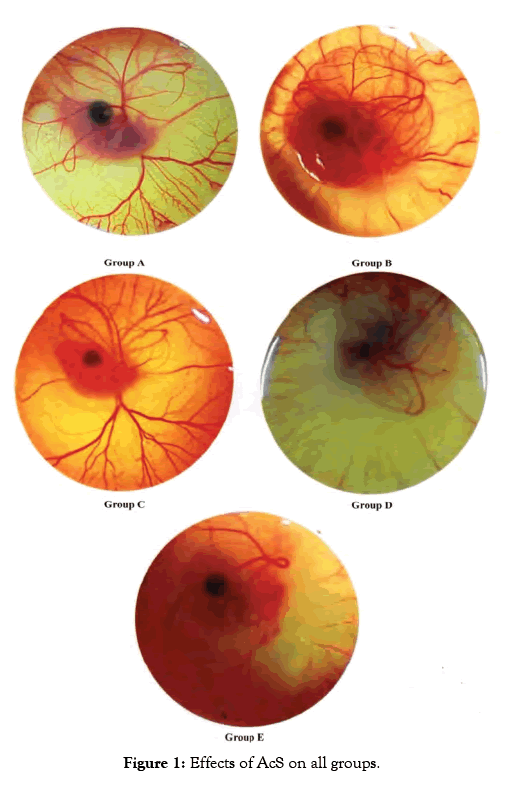
Figure 1: Effects of AcS on all groups.
Grey scaling
Grey image-processing program was used for images of angiogenesis to acquire a much qualitative analysis of angiogenesis and antiangiogenesis, as shown in Figure 2.
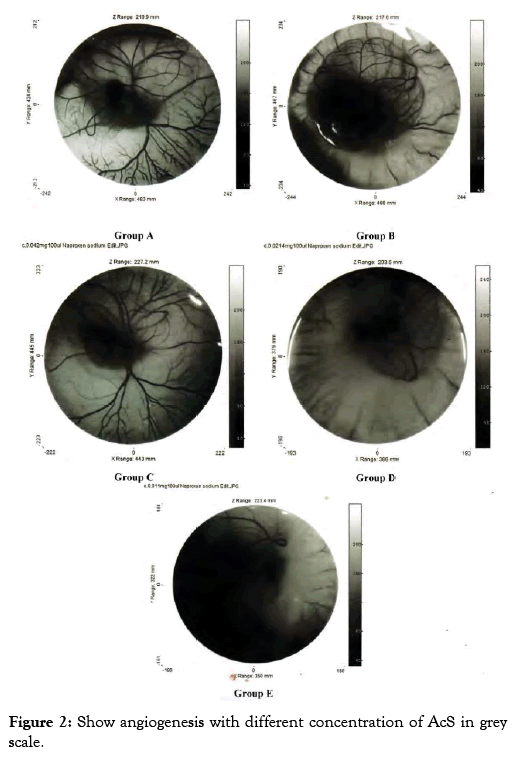
Figure 2: Show angiogenesis with different concentration of AcS in grey scale.
Quantitative analysis
Quantitative analysis was performed diameter of blood vessels, these are discussed as under.
Diameter of blood vessels
A software SPIP was used to determine the vessel diameters of the captured images. Different segments in a selected area for measurements were identified. The respective diameters of all vessel portion located in different segments were identified using the SPIP. Using this procedure, the diameter of a specific vessel was determined automatically at each time point respective drug concentration. The distribution of the vessel diameter of the CAM was observed to be normal. That is obtained from the results; it was obvious that the vessel diameters of the CAM did not change significantly over time. It was also observed that in Group A and B diameter increased which shows that AcS increases diameter in higher concentration. However, there is not significant statistical difference between both groups. On the other hand, group C, D, E at lower concentration AcS worked as an anti- angiogenic agent and diameter of blood vessels decreased with a significant difference among control and treated group shown in Table 1.
| S. No | Name of group | Concentration of drug | No of eggs |
|---|---|---|---|
| 1 | A | PBS 0.1 ml (Control group) | 5 |
| 2 | B | 0.64 mg/0.1 ml AcS | 5 |
| 3 | C | 0.32 mg/0.1 ml AcS | 5 |
| 4 | D | 0.16 mg/0.1 ml AcS | 5 |
| 5 | E | 0.08 mg/0.1 ml AcS | 5 |
Table 1: Experimental groups with respective concentrations and number of eggs.
In angiogenic study CAM assay is broadly used and is specifically will suitable for the diseases study differentiate via proliferative retinal vasculature, for example age related muscular degeneration [7]. In my research work, this assay was used in order to evaluate the angiogenic and anti- angiogenic outcome of AcS and to evaluate the effective dose of AcS for angiogenesis. Multiple treatments for anti-angiogenic drugs are required and, as one can imagine, the advantages of a less invasive mode of drug administration would be great. In the experimental model, the inhibition of the physiologically developing CAM membrane is investigated by applying different concentration of AcS. The treatment of the CAM with anti angiogenic compound in the phase of development characterized by exponential vascular growth inhibits the natural development of capillaries and vessels that indicates the antiangiogenic properties of the AcS. The result of this drug is shown in Figure 1. It shows that AcS is angiogenic at higher concentration and anti-angiogenic at low concentration. Grey image-processing program were used for images of angiogenesis to acquire a much qualitative analysis of angiogenesis and anti-angiogenesis, as shown in Figure 2, similar studies were performed by Blacher et al. [8]. They used new advances image investigation to quantify CAM angiogenic response from optical microscopic interpretations, covering components, from the large supplying and feeding vessels down to the capillary plexus. Their morphometric investigation highlighted that a precise quantification of the CAM vasculature desires to be executed at several scales [9]. Quantitative analysis was performed including imaging studies, and diameter of blood vessels Figure 3. After applying AcS on CAM images were captured at regular time intervals. The images captured were then analysed with the software SPIP. There were transformed in to three dimensional views at 90° top and 45° as shown in figure respectively. These 3D views are giving better understanding of blood vessels network. Similar studies were performed by Hussain et al. [10]. Quantification of diclofenac sodium on angiogenesis by using Chorioallantoic Membrane (CAM) Assay. Fourteen parameters of 3D surface roughness were additionally assessed for evaluation. Use of diclofenac sodium on CAM at day six of hatching (0.7% centralization of diclofenac sodium) indicated hostile to angiogenic impact. Comes about indicated changes in engineering of CAMs, diminishing of essential, auxiliary and tertiary veins, lessening in surface unpleasantness parameters, increment in kurtosis of surface, and abatement in Abbott bend [10]. Software SPIP was used to determine the vessel diameters of the captured images Figure 3. Different segments in a selected area for measurements were identified. The respective diameters of each vessel portion located in different segments were identified using SPIP. Using this procedure, the diameter of a specific vessel was determined automatically at each time for the respective drug concentration. The dispersal of the vessel diameter of the CAM was observed to be usual. From the investigation, it was obvious that the vessel diameters of the CAM did not modify meaningfully over time. It was also observed that in group A and B diameter increased which shows that AcS increases diameter in higher concentrations. However, there is not significant statistical difference between both groups. On the other hand, in low concentrations, AcS worked as an anti-angiogenic agent and diameter of blood vessels decreased with significant differences among control and treated groups as shown in Table 1. Another study was conducted by Salas et al. to assess the phytochemical components and possessions of herbal plant abstracts, such as Gynura nepalensis, Pandanus odoratissimus L and Carmona retusa masam, as likely angiogenesis inhibitors using the CAM analysis [11]. They found similar reduction in diameter with Carmona retusa masam [12-17]. The present study was also performed to investigate that blood vessels are formed by the selection of capillaries in the transformation of a capillary network to a branching system in the wall of quail of yolk plexus [18-21]. To recognize the method of development of branching arrangements of blood vessel transformation, a series of photographs was recorded with a computer simulation of the process of in vivo vascularization [22-25]. The simulation established that a positive feedback system contributed in the development of a branching pattern. As the body of an embryo grow, it was witnessed that in Group A and B, there was fork branching pattern while in group C, D and E, there was polygonal are shown in Figure 2. An area where the progress was rapidly got much blood flow and produce very fine networks of capillaries [26-29]. Finally it should be noted that it is complicated to translate the concentrations or doses applied to the CAM into doses administer in clinical applications. This is due to the following difficulties; the method of drug administration in clinical applications varies with the methods used in the research protocol, which results in different absorption and bioavailability of the drugs. There is effectively an overall lack of information concerning serum and blood concentrations of drugs, and there are important difficulties in translating between a chicken and human [30-31]. This model and this research therefore are not being used to determine exact effective concentrations or to make a direct translation to clinical application, but instead serve to determine general trends and interactions of models and drugs in order to determine, if these therapies are viable treatment options which should be investigated in more depth [32-34].
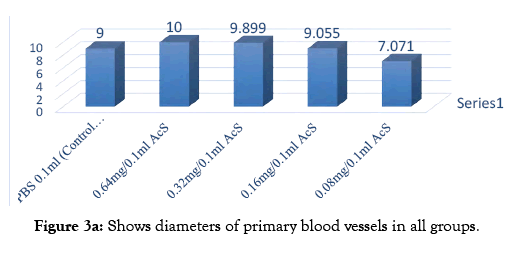
Figure 3a: Shows diameters of primary blood vessels in all groups.
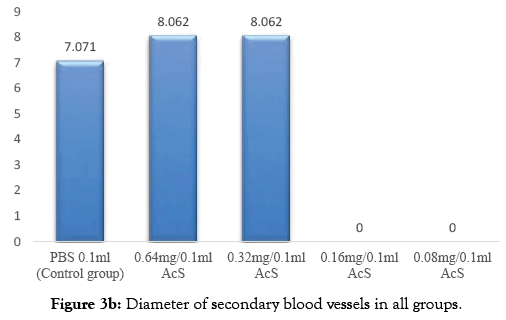
Figure 3b: Diameter of secondary blood vessels in all groups.
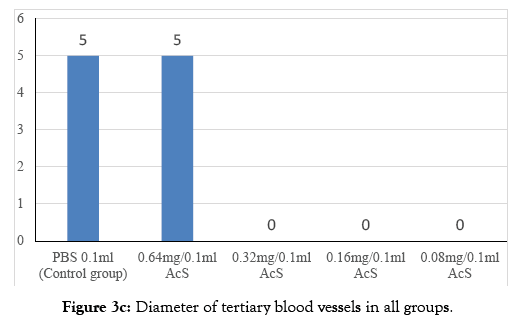
Figure 3c: Diameter of tertiary blood vessels in all groups.
It is concluded that presentation of AcS on CAM at 6th day of incubation exhibited angiogenic effects at high concentrations (0.64 mg/0.1 ml of AcS) and anti-angiogenic effect at low concentration (0.08 mg/0.1 ml of AcS). Results indicated noticeable modifications in design of CAMs, thinning of primary, secondary and tertiary blood vessels. AcS shows dual and dose dependent effect. This indicates that AsS can be helpful for development of tactics for prevention and therapy for different types of angiogenesis dependent diseases.
Citation: Nazeer A, Shakir L, Rahman Z, Omer O, Najam K, Choudhary A, et al. (2020) A Study to Determine Effects of Aceclofenac Sodium on Angiogenesis by using Chorioallantoic Membrane (CAM) Assay. J Appl Pharm 12: 271. doi: 10.35248/2376-0354.20.12.271
Received: 11-Nov-2019 Accepted: 25-Jan-2020 Published: 03-Feb-2020 , DOI: 10.35248/2376-0354.20.12.271
Copyright: © 2020 Nazeer A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.