Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Case Series - (2020)
Background: Soft-tissue filler injections are amongst the most widely used treatments in nonsurgical facial rejuvenation. Although generally deemed safe, rare catastrophic side effects are regularly reported in the literature, the most devastating of which is irreversible blindness. The avoidance of these serious vascular events is of the utmost importance during soft tissue filler injections.
Objective: This article suggests a procedural protocol to greatly minimize the risk of embolization of blood vessels with soft-tissue fillers.
Discussion: Extensive cadaver studies have shown significant inter-individual and intra-individual variations in vascular anatomy. Hence, detailed knowledge of anatomy, albeit very important, is not always sufficient to prevent the accidental cannulation of major arteries. Furthermore, the recent inclusion of xylocaine, a known vasodilator, in several proprietary filler substances, may increase the probability of intravascular injection. Hence, avoidance remains the single most important factor in preventing the dire outcomes of vascular complications with filler substances.
Conclusion: The outlined treatment recommendation, if diligently followed, may greatly minimize the risk of intraluminal injection with soft-tissue fillers.
Filler injections, Embolization, Soft tissue, Blood vessels
According to the American Society of Plastic Surgery, soft-tissue filler injections comprise the second most popular minimally-invasive procedure for facial rejuvenation, with 2.7 million procedures performed in 2019 in the USA.
The vast majority of filler injections are performed with hyaluronic acid; however, other soft-tissue fillers including calcium hydroxyapatite, poly-l-lactic acid, polymethylmethacrylate, and fat are also used to augment volume in the mid-face, lips, chin, cheek, jawline and are also used for rhinomodulation, an increasingly popular procedure for nonsurgical nose reshaping [1-3]. Although soft-tissue fillers are generally believed to have a positive safety profile, serious adverse reactions have been reported. The most devastating side-effects are those associated with vascular occlusion, due to the accidental intravascular injection of the filler substance with resultant embolization of the vessel. Several catastrophic consequences may occur, including skin necrosis, stroke, vision impairment, and blindness.
In fact, the very first report in the literature was in 1906, when Brawley reviewed several cases of blindness due to paraffin injections for saddle nose [4]. More recent surveys of the literature have found that vision impairment and irreversible blindness have been associated with the full spectrum of soft tissue fillers used in aesthetic medicine [5-7]. However, it has been shown that vascular events are more frequent and more severe with non-hyaluronic acid fillers such as calcium hydroxylapatite and polymethylmethacrylate as compared to hyaluronic acid fillers. Furthermore, hyaluronic acid-related blindness is likely to have better outcomes than nonhyaluronic acid fillers due to hyaluronidase enzyme use to degrade the filler [8].
A review of the world literature on blindness resulting from filler injections from 1906 to 2015 revealed only 98 cases, and an update looking at data from 2015 to 2018 revealed a further 48 cases worldwide [9,10]. Hence, although these figures suggest that vision loss caused by soft tissue fillers is an infrequent occurrence, it is believed by many that these incidences are greatly underreported. Typically, most often, it is someone other than the operating physician who reports such life-altering phenomena resulting from filler injections and, unfortunately, descriptions in the literature of the exact circumstances leading to the vascular event, such as the mode of injection, are scant.
The injection sites most associated with filler-induced vision loss are the glabellar complex, the nose, forehead, and nasolabial fold. However, other areas also reported to be implicated include the temple, cheek, periorbital area, eyebrow, and chin [10,11]. Hence, although there is an obvious danger triangle within the mid-face, no anatomical area is deemed totally safe.
The face is highly vascularized, and the arterial subdermal plexus of the face is supplied by both the internal and external carotid arteries. The periorbital area receives its blood supply mainly from the internal carotid system, and the mid and lower face receives blood supply from the external carotid artery. However, there are several connections or anastomoses between the internal and external carotid systems [12]. The ophthalmic artery is one of the major intracranial anastomoses between the external and internal systems. The ophthalmic artery has thirteen branches and several of the facial arteries, including branches of the internal carotid system, the supraorbital, supratrochlear, and dorsal nasal arteries, are distal branches of the ophthalmic artery. The retinal artery and posterior ciliary arteries are proximal branches of the ophthalmic artery. Injection of filler directly into the lumen of one of the branches of the internal carotid system or one of the anastomoses of the branches of the ophthalmic artery with the external carotid system, such as the angular artery, superficial temporal artery, and anterior deep temporal artery can lead to serious vascular complication [12,13] (Figure 1).
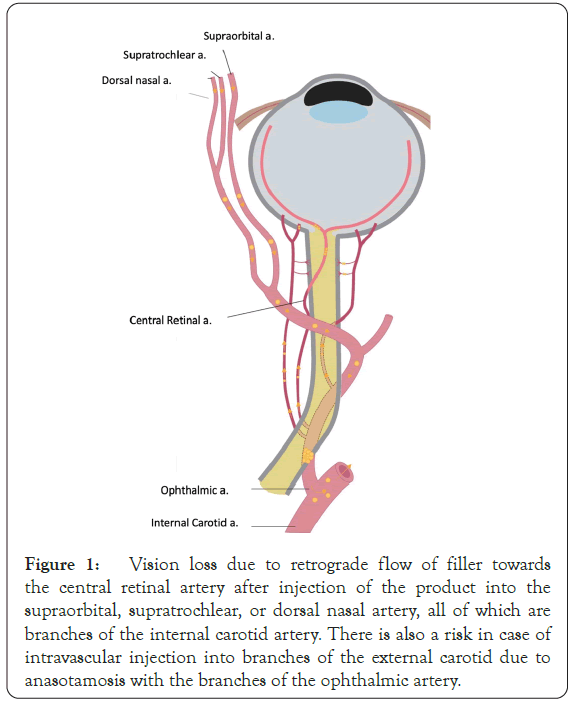
Figure 1: Vision loss due to retrograde flow of filler towards the central retinal artery after injection of the product into the supraorbital, supratrochlear, or dorsal nasal artery, all of which are branches of the internal carotid artery. There is also a risk in case of intravascular injection into branches of the external carotid due to anasotamosis with the branches of the ophthalmic artery.
During injection, pressure on the plunger of the syringe propels the filler into the cannulated vessel. Once the pressure is released, the systolic blood pressure propels the filler retrograde into the ophthalmic artery branches. Consequently, even a minute amount of filler inflowing into the central retinal artery is sufficient to cause permanent blindness. The loss of vision in the affected eye usually occurs within seconds after the injection and may or may not be associated with pain and headache [14]. Stroke may also occur through the retrograde filling of the internal carotid artery. To date there are no proven protocols to reverse filler-induced vision loss.
There is no doubt that anatomical knowledge is very important in the avoidance of vascular complication. However, several recent detailed cadaver studies have highlighted the fact that there are significant differences in facial arterial anatomy between individuals and even between both sides of the face in the same person. Nonetheless, it appears that there are less inter-individual variations for the depth at which the facial arteries are located [15]. Hence, this variability predisposed to a high probability for unintentional intravascular injections by even the most experienced injectors. To avoid accidental intraarterial injections, the practice of blunt cannula use has become increasingly popular. The premise is that blunt cannulae cannot pierce through vital structures such as arteries and nerves and are much safer than needles. Although this is true, recent cadaver studies have uncovered the "darker side" of cannula use. It has been shown that a 25 G cannula can penetrate an artery in certain situations where the cannula is at a perpendicular angle to the artery [16].
A retrospective review was performed of over 10,000 patient records from January 2010 to May 2017. The patients had received filler treatments in our clinics in Dubai, using a protocol to avoid intravascular injections, termed "Safe Filler Injection Protocol". An average of 2 syringes of soft tissue fillers per patient was used, resulting in a total of over 20,000 syringes. Various fillers were used including hyaluronic acid, calcium hydroxylapetite and polycaprolactone-based fillers. Patients presented two weeks after the initial treatment session for a follow-up consultation. The treatment results were documented by obtaining post-treatment photographs (Canfield Vectra 3D Imaging system, Canfield Scientific, New Jersey, USA). The outcomes of the treatment, including any untoward effects, were documented on the patient's electronic treatment notes (Capsule, Tendercare, Dubai, and UAE).
Safe filler injection protocol
This comprises four key steps
1. Strict avoidance of filler injections to glabella and nose, which are very high-risk areas that receive their blood supply from direct branches of the internal carotid system.
2. Injection of fillers to the correct, safe depth to avoid major blood vessels (Table 1).
| Area | Depth of injection |
|---|---|
| Temples | Superficial subdermal layer |
| Mid face | |
| Tear trough | Supra periosteal |
| Pre maxillary space | |
| Zygomatic arch | Supra periosteal |
| Nasolabial fold | |
| Deep pyriform fossa | Supraperiosteal |
| Nasolabial fold | Superficial subdermal |
| Submalar and pre-auricular area | Subcutaneous layer |
| Mandibular border | |
| Prejowl sulcus | Subcutaneous or supraperiosteal |
| Post jowl | Subcutaneous |
| Mandibular angle | Subcutaneous or supraperiosteal |
| Chin | Subcutaneous or supraperiosteal |
Table 1: Safe injection depth to avoid major blood vessels in each facial zone [15,17].
3. Infiltration of high-risk areas, such as the temples, infraorbital area, pre-maxillary space, and deep pyriform fossa, with 2% xylocaine solution containing 1:80.000 adrenaline. The infiltration of anaesthetic solution followed by applying ice pads around 10 minutes before injection, allows for effective vasoconstriction of the blood vessels in the treatment areas.
4. Use of a 22 G × 50 mm blunt-tipped cannula almost exclusively in all of the treatment areas.
The strategy of injecting at the correct depth in each treatment area to avoid major arteries and injecting the filler with a large blunt-tipped cannula in tissue where the blood vessels have been previously constricted with adrenaline greatly improves safety and reduces the chances of intraarterial cannulation.
The retrospective review of the case series of over 10,000 patients who had been injected with various types of fillers using the "Safe Filler Injection Protocol" showed absolutely no incidence of vascular complications. A further advantage of the protocol was that it improved patient comfort and reduced the incidence of minor side effects such as bruising and oedema.
Unintentional intraarterial injection of soft tissue fillers, albeit a rare occurrence, may lead to the interruption of the blood flow with devastating consequences for both the patient and the physician.
Since the position of facial arteries is not entirely predictable, with various inter and intra- individual differences, detailed knowledge of vascular anatomy does not necessarily ensure the avoidance of intravascular injection.
Recently, new-generation hyaluronic acid and calcium hydroxylapatite-based fillers have been developed containing the local anesthetic xylocaine, intended to decrease discomfort and pain during injection [17,18]. Xylocaine is a known vasodilator; hence, with every thread of filler deposited within the tissue, the potential for dilating the vessels within the treatment area increases, as does the risk of accidental deposition of filler within the dilated vasculature. These new generation fillers, containing xylocaine, constitute an additional predisposing factor to intravascular injection.
Although there is no doubt that cannulae, particularly large bore cannulae, are safer than needles for filler injections, there is evidence to show that even blunt-tipped cannula may penetrate arteries [15,16]. Hence it is warranted to institute every possible measure to avoid the intravascular deposition of fillers.
The treatment of over 10,000 patients with soft-tissue fillers, using the “safe filler injection protocol” did not result in any cases of vascular complications. Furthermore, even minor complications such as bruising and oedema, were rarely seen. The “Safe Filler Injection Protocol”, greatly reduces the probability of intraarterial injection since fillers are injected at the correct depth to avoid the major vessels and large-bore cannulae are used in tissue pre-treated with the vasoconstrictor, adrenaline. Hence the risk of cannulating a constricted blood vessel with a large bore cannula is highly unlikely.
There is no doubt that soft tissue filler injections are invaluable tools within the spectrum of aesthetic treatments, however, they are associated with rare serious vascular complications.
The devastating consequences of intra-vascular injection of soft- tissue fillers are a matter of great concern. It must be stated that unlike other soft tissue filler-induced adverse events, which may be filler or patient-related, such as granulomas or hypersensitivity reactions, the consequences of intraarterial injections are purely practitioner induced. Hence soft-tissue filler injections must be performed with the utmost caution on the part of the practitioner. Every possible measure must be taken to avoid the life-altering consequences of intraarterial occlusion for both the patient and the injector. In our experience, the “Safe Filler Injection Protocol” is very effective in avoiding vascular complications.
Citation: Khattar MA (2020) A Procedural Approach to Minimize the Risk of Embolization of Blood Vessels with Soft-Tissue Fillers. J Clin Exp Dermatol Res. 11:544.
Received: 01-Dec-2020 Accepted: 16-Dec-2020 Published: 23-Dec-2020 , DOI: 10.35248/2155-9554.11.s7.544
Copyright: © 2020 Khattar MA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.