
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2020)Volume 10, Issue 5
Background: Development of new drugs to cope with emerging and existing diseases resistant to current treatment
regiments needs rapid, structured clinical evaluations of such therapies in suitable clinical trial subjects. Nigeria,
resource poor country, is trying to foster the field of clinical research to strengthen its medical and healthcare
capabilities.
Material and Methods: A cross-sectional descriptive study focusing on human resource capacity for clinical trials,
fitness of private medical practices as trial sites and therapeutic areas of interest of private medical practices in Cross
River State, Nigeria was performed. We randomly selected 66 private medical practices. We administered close ended
questionnaire. Twelve of these 66 were further selected based on their geographical location in Cross River, Nigeria,
for three focus group discussions of 4 medical directors each. Two members of the regulatory authorities and
institutional review board in Cross River State had in-depth interviews conducted by the authors.
Results: Six (9%) of medical directors of the private medical practices had ever participated in clinical trial study and
only 17 (26%) of the practices had ever published in an academic journal. Fortunately, this result showed that over 64
(97%) of these private medical practices were highly desirous of participating in clinical trials and 65 (98%) desired to
publish or co-author original articles in reputable academic journals. High percentages of the practices had interest in
therapeutic areas across predominant diseases such as cardiovascular, malaria, respiratory, diabetes, HIV and testing
of new medical devices.
Conclusion: Majority of private medical practices were well equipped to conduct and highly desirous to participate in
clinical trials in Cross River State, Nigeria. Further studies with larger cohort and more emphasis on the ICH-GCP
guidelines, specific training of the investigators and the staff are warranted.
Clinical evaluations; Cross river state; Cardiovascular; Medical devices; Nigeria
Global disease burden is increasing rapidly as several life styles and communicable diseases are increasing [1]. In this scenario, countries like Nigeria are also poised to ramp their efforts to have adequate, efficacious and accessible medicines at affordable cost. The total value of Nigeria pharmaceutical industry is estimated to worth $1.3billion, accounting for less than 0.25% of Gross Domestic Product (GDP) [2]. There are a total of 132 local drugs manufacturing companies in Nigeria licensed to operate in the industry. Only four of these 132 local manufacturers have obtained the World Health Organization (WHO) certification of Good Manufacturing Practice (GMP), making only these four fit for international market and competition. The challenge of increasing foreign exchange expressed in increased cost of imported drugs, up to 200%, makes it expedient to support the demand for made-in-Nigeria drugs. A number of clinical trials to assess the efficacy of new drugs are being conducted in Nigeria. Although not all trials are registered by the clinical trial registry, over 377 trials have been documented to have had their outcome published by members of International Committee of Medical Journal Editors (ICMJE) [3]. There are over 72 tertiary hospital centres for conducting clinical trials in the country. With this increase in demand for clinical studies across different phases, there is acute need to ramp up efforts to cope with the huge demand for clinical trials.
In Nigeria, academic healthcare facilities, tertiary care hospitals, particularly university teaching hospitals, have long been perceived as the most acceptable, reliable and main domain of clinical research activities. Research grants and funding for clinical trials are predominantly concentrated in university teaching hospitals and other tertiary healthcare facilities in the country [4]. This trend has been consistent despite changes in healthcare behaviors of patients. The country has witnessed an increased number and wide distribution of private health facilities in all local government areas. There is palpable increase in the quality of private medical practices [5,6]. Fortunately, this improved image of private medical practice is coming at a time when there is dwindling public health resources and infrastructures in the tertiary health institutions and progressive micro and macro-economic down turn of public health facilities in Nigeria. In Cross River state of Nigeria, there is only one tertiary public hospital but well over 100 functional private hospitals. Presently, only a few clinical research activities are carried out in private medical practices in Nigeria as against over 72 public tertiary research centres. Most of these private practices are mainly service oriented and are focused on satisfying their patients in promptness and quality of health service delivery. Clinical trials in some of the practices are considered unnecessary distractions.
Generally, clinical trial activity in Nigeria is still at very low ebbs [7-9]. In 2018, NAFDAC inspected only 13 clinical trial sites for the conduct of sponsored clinical trials. All these sites were tertiary public hospitals. Presently, only a few clinical research activities, less than 30, are known to have been carried out in private medical practices in Nigeria. No trial research has been registered by a private medical practice with the Nigerian clinical trial registry. This is because most of the country’ s private practices are mainly service oriented and are focused on satisfying their patients in promptness and quality of health service delivery. This is influenced by the fact that private practitioners had in the past psychologically considered trials to be academic exercise that was confined to a few tertiary hospitals in the country.
In Nigeria, there is increasing patients ’ patronage of private medical practices for healthcare services following accreditation of private medical practices by National Health Insurance Scheme (NHIS) for treatment of people in the formal sector of the economy. It is documented that 65% of all patients treated in hospitals in Nigeria are treated in private medical practice. To further enhance this fact over 70% of healthcare facilities accredited and registered by National health Insurance Scheme in Nigeria are private medical practices. Already, the population of informal sector employees comprising small scale businesses and companies, industries, ventures and enterprises is cared for by private medical practices. Consequently, there is a huge number of willing clinical trial participants laying uninvolved in the volume of clinical researches conducted in Nigeria because these are limited to public and academic Institutions. In 2018, 13 such sites were on going as earlier stated above. In other climes, clinical trials are increasingly conducted in private medical practices. This enables trials to recruit high numbers of eligible subjects within the trial target time. Private medical practices in those climes are considered as partners rather than competitors to quality of healthcare delivery and use of innovator drugs for patients ’ care. With this prudent background and unmet medical need to conduct more and more clinical studies, we investigated the preparedness of private medical practices to conduct clinical trials across different therapeutic areas in Cross River state, Nigeria.
Study design
This study was a prospective cross-sectional descriptive with analytical component in design. This research was made to comply with any law(s) and regulation(s) addressing the conduct of clinical research in Cross River State specifically and Nigeria in general. The research sort the affirmative approval decision of Cross River State Health Research Ethical Committee (CRS-HREC), that the research proposal had been reviewed and could be conducted at the participant facility sites within the constrains set forth by the HREC, the health facility, Good Clinical Practice (GCP) and the applicable regulatory requirements.
Cross River State is home to an academic hospital, the University of Calabar Teaching Hospital, Calabar. There were 22 other in-patient public health facilities, comprising 2 hospitals run on Public Private Partnership (P3) contract, 5 faith base facilities made up of 3 general hospitals and 2 specialist care centres for tuberculosis and leprosy. There were 15 other general hospitals wholly owned and run by the state government as general/public hospitals, unevenly distributed across the 3 political senatorial districts of the state. There were 85 private medical practices headed and managed by their proprietors. These proprietors were qualified medical practitioners, licensed to operate as private–for–profit general or specialist practices. These medical entrepreneurs were referred to as Medical Directors (MD) of their practices. They are held responsible for any lapses or failure in the quality of medical services by regulators of healthcare services in the country. Theses MDs were the principle investigators (PI) of clinical trials in their facilities. They are responsible for the day-to-day provision of clinical services and any clinical trial that could take place in their practices.
Eligibility criteria
Eligibility for participation in this research by private medical facilities was limited to private medical facilities accredited and registered by the Cross-River State ministry of health as a provider. These must also be fully accredited and registered as a primary or secondary provider by the National Health Insurance Scheme (NHIS). The medical facilities that had these accreditations but currently do not have any qualified medical doctor practicing in the facility were not eligible to participate.
Recruitment
Recruitment was by words of mouth and advertisement at Association of General and Private Medical Practitioners of Nigeria (AGPMPN) seminars and other professionals ’ stakeholders’ workshops and conferences. One-on-one contacts were made with medical directors of the private practices in their facilities.
Questionnaires survey
A survey with questionnaire consisting 25 questions was conducted with the medical directors of private practices as subjects from 6th August 2019 to 28th August 2019. This was some week behind the scheduled period because of the delay experienced in obtaining ethical approval from Cross River State-Health Research Ethics Committee (CRS-HREC).
Focus-group discussion
Focus-group discussion was conducted for groups of participating private practices within each of the three senatorial districts of Cross River State, Nigeria. Though there was intension to include at least one Clinical Research Organization (CRO) in the discussion group, there was no CRO available in the state.
In-depth interviews
These recruited participants from Cross River State health research ethics committee (IEC), National Agency for Food and Drugs Administration and control, Association of General and private Medical Practitioners of Nigeria (AGPMPN) and university of Calabar teaching hospital institutional ethics committee. There were no clinical trials sponsors available in Cross River State intended for interview.
Participants
Participants were by simple random sampling selected from the medical directors (MD) of the 85 registered private medical practices. Participants for in-depth interview represented Cross River State health research ethics committee (CRS-HREC), National Agency for Food and Drugs Administration and control, Association of General and private Medical Practitioners of Nigeria (AGPMPN). Participants for focus group discussion were 12 MDs, four representing each of the three geopolitical regions of Cross River, Nigeria, referred to as senatorial district.
Data collection method
Data collection for this cross-sectional study was by questionnaire. Structured questionnaire tool was a restricted or closed form for private medical practitioners with short questions. Audio records of focus group discussion and data from in-depth interviews were done.
Self-administered questionnaire, focus group discussion, indepth interview was conducted and captured, recorded on paper base format.
Sample technique
A Simple Random Sample technique was applied. This was suitable because the sample frame was small, about 100 -130 private medical practices in the state. Alternatively, a one-stage cluster sampling to represent the three geopolitical regions of the state (North, Central and South senatorial districts of the state) could have been done.
Sample size
This was estimated by application of the formula 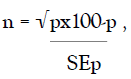 where the standard error of percentage (SEp), or proportion for
the proposed simple random sample method is derivable from a
percentage estimate of 50% (p = .50), a confidence interval of
5%(.05) and confidence level of 95%.
where the standard error of percentage (SEp), or proportion for
the proposed simple random sample method is derivable from a
percentage estimate of 50% (p = .50), a confidence interval of
5%(.05) and confidence level of 95%.
Data analysis
Descriptive statistical analysis was applied to provide explanations and further evaluations of some objectives and problems of the records.
This research revealed the state of preparedness of private medical practices in Cross River State, Nigeria, to incorporate clinical trials into their routine care practices. Here presented is result of 10 areas affecting clinical trial execution in private practices. These are (i) human resource capacity, (ii) ethical issues, (iii) facility fitness as trial site, (iv) therapeutic areas of interest, (v) documentation, (vi) perceptions about clinical trial (CT), (vii) motivational factors of private-sector involvement, (viii) steps-forward to becoming a clinical trial site, (ix) challenges, and (x) ready and fit to start trials across the state. A total of 66 private medical practices participated in the survey. Six of these were in the geopolitical part of the state referred to as northern senatorial district; ten were in the central senatorial district while 50 were in the southern senatorial district. There were three focus group discussions, each made up of four persons and a total of twelve participants. Two In-depth interviews were conducted. This study was conducted in the months of August to November 2019.
When asked if the MD had participated in any clinical trial, 90% responded that they never done so, 74% had never published in any journal. Over 90% expressed willingness to participate as well as publish in medical/research journals. Fifty (75.6%) private medical practices out of 66 indicated readiness to hire registered nurses, and equal number of 50 (75.6%) of the 66 participating practices indicated readiness to employ research coordinators. Fourteen 14(21%) of the practices would wish to employ pharmacists while 20 (30%) of these 66 private practices were ready to engage the services of laboratory scientists. A total of 26 would hire medical doctors to assist in their research teams. In response to question on use of existing Institutional Review Board (IRB) in the Cross-River State, 34 (51.5%) private facilities opted for Cross River State Health Research Ethical Committee (CRS-HREC) for the review of protocols of sponsors of researches to be conducted in their facilities.
Table 1, describes the various choices for the therapeutic area preferences, where 59 (89%) of the private medical practices included in this survey indicated interest on conducting or participating in the conduct of clinical trials in diabetes therapy. Equally 56 (85%) of the private medical practices involved in this survey signified their interest in clinical trials of medical devices. Hypertension therapy had 49 (74%) of the practices interested in it while (61%) 40 were interested in cancer therapy. Malaria, infectious diseases and HIV had 35 (53%), 42 (64%) and 39 (59%) of the participating private medical facilities interested in their therapies. Tuberculosis, liver diseases, diseases of gastroenteritis were identified as therapeutic areas of special interests by 24 (36%), 22 (33%) and 26 (39%) respectively of the private medical practices in Cross River State that were interviewed in this research. Sickle cell and respiratory diseases therapeutic areas were selected by 37 (56%) each of the private medical practices desiring to incorporate clinical trials into their normal/routine general practice. The patient population served by these private practices were assessed, the details are provided in Table 2. Three facilities 3 (4.5%) of the facilities interviewed had over 50,000 patients each registered for care while a total of 44 (167%) cared for well over 10,000 patients registered in each of the facilities. Eleven of the private practices interviewed had fewer than 5,000 patients each in their care. To identify among the practices that participated in this research, which of these had space available for clinical trial services, 63 (96%) alluded to having space and agreed to give out the space for clinical Trial services in their practices. Only 3 (4%) were uncertain of availability and provision of space for clinical trial activities in their practices.
| Area | Ma | I.D | Dia | Hyp | R.D | HIV | S.S | Ga | Ca | L.D | TB | NMD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of practices | 35 | 42 | 59 | 49 | 37 | 39 | 37 | 26 | 40 | 22 | 24 | 56 |
Ma: Malaria; ID: Infectious Diseases; Dia: Diabetes; Hyp: Hypertension; RD: Respiratory Diseases; SS: Sickle Cell Disease; Ga: Gastrointestinal Diseases; ca: Cancers; LD= Liver Diseases; NMD: New Medical Devices
Table 1: Therapeutic areas of interest.
| Patient population per practice | Less | 1,000-3000 | 3,000 – 5,000 | 5,000 – 10,000 | 10,000-20,000 | 20,000-40,000 | 40,000-50,000 | Above 50,000 |
|---|---|---|---|---|---|---|---|---|
| 1000 | ||||||||
| No of practices | 4 | 7 | 11 | 1 | 27 | 12 | 1 | 3 |
Table 2: Practices patients’ population as criteria for trial site.
Interview on the current available methods of documentation in use by the private medical practices that participated in this research revealed that no practice uses electronic medical record (EMR) solely, but 24 (36%) practices use combined electronic medical record and paper-based, while 42 (64%) used paperbased medical records solely.
The specific responses to the question # 12 to 25 of the 26-point questionnaire are listed in the Table 3. Another perception of clinical trial by medical directors of the practices interviewed showed that 41 (62%) private practices disagreed with the notion that clinical trials in private medical practices may push the practices into unrecoverable expenses; 24 (36%) were uncertain and only one medical director believed Trials, make practices to run into unrecoverable expenses. An interview on the financial gains of clinical trial by private medical practices revealed 46 (70%) practices indicated that clinical trial could have a positive multiplier effect on their income, while 19 (29%) were uncertain of the positive effect and 1% disagree that clinical trial could positively affect their income stream. Forty-six (70%) of the facilities that responded in the question inquiring to know if there exists enough reward to warrants the potential risks involved in conduct of clinical Trials in their private medical practices agreed positively while 20 (30%) were not certain. On whether the physicians in this research will agree to conduct clinical trials in their private medical practice, 65 (98%) out of the 66 strongly affirmed their willingness to accept clinical trials in their practices. Three benefits to the private practitioner from clinical trials were deduced and agreed by central senatorial focus discussion group. These were itemized as; a) direct benefits from funds provided by sponsors of the trial b) indirect benefit resulting from multiplier effect of advertisement of trial services of the hospital c) public image enhancement of the practice and private practitioner as a principal investigator.
| Question | Agree | Uncertain | Disagree |
|---|---|---|---|
| (N=66) | (N=66) | (N=66) | |
| Clinical Trial an exclusive preserve of academic and public centres | 13 | 2 | 51 |
| Clinical Trial may make your practice run into financial expenses you cannot recover | 1 | 24 | 41 |
| Clinical Trial could have positive multiplier effect on the income of the practice | 46 | 19 | 1 |
| Clinical Trial require dedicated trained staff | 64 | 1 | 1 |
| Clinical Trials add intellectual stimulation and variety to the daily tasks of seeing patient | 62 | 2 | 2 |
| Do you know that conducting clinical trials in your practice, you will be subjected to regular checks by regulating authorities, monitors and sponsors | 66 | 0 | 0 |
| Would you want to upgrade your practice to qualify as a clinical site? | 64 | 2 | 0 |
| Are you aware that the choice of your practice as a clinical site is dependent on the kind of trial and decision of the sponsor? | 55 | 10 | 1 |
| Would you agree and willing to spend time on the additional training needed to participate in conducting trial research? | 63 | 3 | 0 |
| If clinical trials are conducted in your practice, will you agree to be involved as a principal investigator or co-investigator | 63 | 3 | 0 |
| Do you see a clinical need in your practice to offer research option for development of new drugs, treatments or devices | 62 | 4 | 0 |
| Do you agree there is enough reward to warrant the potential risks involved in clinical trials | 46 | 20 | 0 |
| Would you agree to conduct clinical trials in your practice? | 65 | 1 | 0 |
| Clinical trials requires space in the facility, would you agree to provide adequate space dedicated for trials in your facility | 63 | 3 | 0 |
Table 3: Table showing responses for questions 12 -25.
Sixty-four (96%) of directors of private medical practices interviewed confirmed they would want to upgrade their practices to qualify as clinical trials site, while 2 (4%) were not sure if they would or not upgrade their practices. The awareness by practitioners that the choice of their practices as clinical trial sites depended on the criteria set by the sponsors was agreed by 55 (83%) of the private medical practices. One (2%) disagreed, and another one practice (2%) did not offer any answer while 9 (14%) were not certain. The Northern senatorial focus group discussants agreed that holistic upgrading of the facility can be achieved within weeks to meet selection criteria of any sponsor. It is noteworthy that a dissenting voice was loud and clear that only large and thriving private practices may have funds in their hands to upgrade facilities without the sponsors ’ assistance. Would private medical practice medical directors (chief physicians) agree to willingly spend their time on additional training needed to offer them the education required to participate in conduct of clinical trials in their practices, 63 (95%) agreed while only 3 expressed uncertainty.
Responding to the questionnaire on principal investigator, 63 (95%) of the private medical practitioners interviewed confirmed their readiness to perform the functions of principal investigators in events, clinical trials were conducted in their practices. However, three practitioners were uncertain if they would actually be ready to perform the principal investigators’ functions in their practices. Geographical representation of respondents to the questionnaire showed equitable distribution of 33 practices from the southern senatorial district, 20 from the central district and 13 from the north.
This study was very unique in many ways. It was the first initiative to assess and evaluate the preparedness of the private practioners to conduct clinical studies in Nigeria. Second, this study revealed a ground reality picture on the interest as well as the current limitation to upgrade the clinical trials conduct scenario in the Cross-River state, Nigeria. Third, if this investigation can be treated as benchmark, it will foster the discipline of clinical trials and or clinical pharmacology in Nigeria. It attempted to identify areas of collaboration between the private medical practices conducting clinical trials and existing academic trial sites. The study evaluated the opinion of clinical research regulators on the emerging demands of clinical trials in private medical practices in Nigeria.
However, beyond this desire by the private practitioners to run clinical studies, the level of their capacities as principal investigators was very low. Capacity based on previous experience in clinical trials participation was appallingly low compared to the strong desires to commence research in private practices. Majority of private medical practice operators have never participated in any single trial.
Fortunately, the determination to participate in clinical trials is expressly backed by the high level of sacrifice of time, space, infrastructures, material, finance and educational studies the number of those wishing to participate in clinical trials in this study had indicated to provide. In an in-depth interview, one of the respondents expressed that “private medical practitioners are business oriented. Not many would want to invest in a venture they are not qualified’’. So, he believed that the practitioner would ensure he or she meets qualification criteria as defined by the trial.
Few private practices have ever published in a standard scientific journal. This generally defined how low the capacity, preparedness, experience and expertise of the private medical practitioners in Cross River State is in clinical trials. Fortunately, this low capacity was seen as one of the strongest motivating factors for advocating clinical trials in private practices. Most private doctors strongly wish to make-up their academics by participating in clinical trials and publishing clinical research results in academic journals of great repute. Having practiced for many years, the private practitioners have worked very hard to get to where they are, running practices with large patient population that matches trials’ inclusion and exclusion criteria. These practitioners have been so long in routine practice that they seek for challenging opportunities to invest their time and stimulate their intellect. They wish to be involved in the conduct of speaking assignments which bring new benefits to their communities. The new challenges of clinical trials in private medical practice would require calibre of staff not previously existing in some of the practices. Registered nurses and health record staff are needed by the private practices that wish to participate in clinical trial. Effective documentation is critical in clinical trials. The practitioners appreciate this and would wish to hire and improve on their staff available to be educated in clinical trials, and to establish a separate staff as meets the standards of a research-based practice.
Private medical practitioners in Cross River State, Nigeria, agreed with the fact that involvement in clinical trials lends their practice the imprimatur of a cutting-edge private practice. Majority of the practices were interested in diabetes, hypertension, infectious diseases and cancer trials. This study found that most medical practices have good number of equipment for the trials in the subject areas of their choice. The Northern senatorial focus group discussants agreed that holistic upgrading of the facility can be achieved within weeks to meet selection criteria of any sponsor. It is noteworthy that a dissenting voice was loud and clear that only large and thriving private practices may have funds in their hands to upgrade facilities without the sponsors’ assistance.
From this study result, majority of private medical practices that participated have large population of over 10,000 from which there are willing patients to participate in trials conducted within the facility. Nearly all the private medical practices in the study run both inpatient and outpatient services. This provides facilities for treatment of subjects with adverse drug reactions or adverse drugs events, serious adverse reaction or events which may occur during the conduct of the interventional clinical studies. This is an important resource available to ensure that the patient’ s safety and wellbeing will be paramount while running those clinical studies.
For quite a long time, clinical research, especially in Africa, has been regarded as a prerogative of the medical academics, tertiary institutions and large public hospitals. In Cross River State, 51 (77%) of private medical practices that participated in this study strongly expressed that clinical trials are not an exclusive preserve of academic medical centres. This huge percentage welcomes incorporation of clinical trial into their private practices as trials are not relegated to public tertiary hospitals only.
From the responses of private practitioners in this research, it was all agreed that clinical trials add to intellectual stimulation and variety to daily tasks of seeing patients. After long years of practice, the need to rejuvenate interest in medical practice could be attained by incorporation of clinical trials in the practice. From the result of this research 49 (74%) of private medical practitioners had never authored or co-authored any publication in a medical academic journal of international repute. Many of such practitioners felt that non-publication as their highest area of deficiency and are motivated to producing good results in clinical trials acceptable by their colleagues for publication in scientific journals of high repute. For these reasons, 65 (98%) of the private medical practices in this research would agree to conduct clinical trials in their facilities. It was interesting to note that 64 (97%) of private practitioners were aware and willing to upgrade their medical practices to qualify as clinical trial sites in their therapeutic areas of interest. As step forward, private medical practices who were wishing to incorporate clinical trials into their routine practices would volunteer to have their principal medical officers, the medical directors, trained as principal investigators. Already 63 (95%) of those who participated in this study had indicated willingness to spend time on additional training needed to participate in the conduct of trial research as principals or co-investigators in their practices.
In focus group 3 discussions, a general opinion was summarized by one of the participants stating thus, “there is nothing wrong conducting clinical trial in private medical facilities. Presently some principal investigators of clinical trials in tertiary academic centres recruit patients and conduct trials in their private practices for the tertiary hospital. What then is wrong for a practitioner who is wholly private and committed to good services in his/her practice from providing clinical trials as a service to the people of the community the practice serves?
A focus group discussion on therapeutic areas of interest agreed that trials are not conducted in a vacuum. Trialists have areas of interests. The general practitioner in Cross River has a vast number of therapeutic areas of interest to research on. So far, lack of sponsorship had limited interest of private medical practitioners from research. It was observed that most often, sponsors concentrated in tertiary academic hospitals, with the notion that Private medical practitioners may not be interested or capable.
This study was not devoid of limitations. Absence of any large pharmaceutical industries in Cross River State that could sponsor clinical trials limited obtaining opinion of sponsors as stakeholders in this research. Although the key author, Dr. Ebaye, had confidence that there was 95% chance that the sample was distributed in the same way as the doctors’ private practices in Cross River state, Nigeria, (standard error=0.05) the sample size was relatively small that the findings of this study cannot be extrapolated to generalize across the entire nation, Nigeria. Also, there was lack of appropriate resources to conduct this study across the country and has to be restricted one state.
In this cross-sectional descriptive study, the preparedness of private medical practices for clinical trial in Cross River State, Nigeria, was established by the availability of the practices’ large patients population, fitness of the private practices as trial sites in various therapeutic areas of study and expressed determination of the practitioners to conduct and publish acceptable trial results in reputable academic journals. Majority of private medical practices were well equipped to conduct and highly desirous to participate in clinical trials. Further studies with larger cohort and more emphasis on the ICH-GCP guidelines, specific training of the investigators and the staff are warranted.
Citation: Ebaye SEN, Jadhav MP (2020) A Cross-Sectional Descriptive Study to Assess the Preparedness of Private Medical Practices to Conduct of Clinical Trials across Different Therapeutic Areas in Cross River State, Nigeria. J Clin Trials. 10:423.
Received: 22-Jun-2020 Accepted: 06-Jul-2020 Published: 13-Jul-2020
Copyright: © 2020 Ebaye SEN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.