Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2024)Volume 15, Issue 5
Background: Glaucoma results in a progressive optic neuropathy characterized by degeneration of Retinal Ganglion Cells (RGC) and their axons. The most recent technique for examining vascular changes in the nerve endings is Optical Coherence Tomography Angiography (OCT-A). OCT-A is a noninvasive imaging technique that uses en-face reconstruction of OCT combined with motion contrast to visualize retinal and ONH microvasculature. The spectrum for primary angle closure disease manifest from Primary Angle Closure Suspect (PACS), and Primary Angle Closure (PAC) to PACG. Pseudoexfoliative Glaucoma (PEXG) is rapid progressive open angle glaucoma with high baseline IOP and wide IOP fluctuation compared to other types of Primary Open Angle Glaucoma (POAG). The vascular function relationship between PACG and POAG is different. In the present study we aimed to evaluate the RPC using OCT-A in primary angle closure suspects vs primary angle closure glaucoma vs PEX glaucoma and to compare the results with normal healthy eyes and to investigate correlations between ONH and retinal vessel density measurements to other structural parameters like RNFL thickness.
Methods: Ultrasonographic pachymetry (CCT), RNFL-OCT, Ganglion Cell Complex (GCC) and OCTA of the microvasculature in the optic nerve head and macula was performed in all patients. The study was conducted on four groups of patients: Primary Angle Closure Suspects (PACS), Primary Angle Closure Glaucoma (PACG), PEXG and normal healthy eyes.
Result: The study population consisted of 24 (10 men and 14 women) primary angle closure suspect eyes (Group A), 25 (13 men and 12 women) primary angle closure glaucoma eyes (Group B), 20 (12 men and 8 women) PEX glaucoma eyes (Group C) and 30 (19 men and 11 women) healthy eyes for control (Group D). The NFL thickness values in all sectors were significantly lower in Groups B and C compared with the normal control eyes in Group D, except for the Superior Temporal (ST) and the Inferior Temporal (IT) sectors which were only significantly lower in the PACG group (Group B). GCC values in the PACG (Group B) were thinner than the PACS (Group A) and control group (Group D). VD in group B and C were significantly lower than those in the PACS (group A) and healthy eyes groups (group D)
Conclusions: The OCTA vessel density was significantly reduced in PACG and PEXG eyes in all circumpapillary sectors and the macula. The wi-VD and cp-VD perform as well as the RNFL thickness for discriminating between healthy and glaucoma. This noninvasive method of RPC vessel density assessment is reliable for detecting glaucoma similar to OCT RNFL thickness. Also, OCTA could be of use for monitoring decrease in RPC VD before glaucomatous optic neuropathy and structural changes have occurred in PAC and PEX eyes
Optical coherence tomography angiography; Primary open angle glaucoma; RNFL; GCC; Deep macular vessel density; Superficial macular vessel density; Primary angle closure suspect
Glaucoma is considered as an irreversible cause of vision loss worldwide [1]. Glaucoma results in a progressive optic neuropathy characterized by degeneration of Retinal Ganglion Cells (RGC) and their axons [2]. The insidious course of glaucoma and often asymptomatic until advanced stages, necessitates the rapid diagnosis, prompt treatment and monitoring of the disease. Studies have acknowledged both high Intraocular Pressure (IOP) and vascular dysfunction as risk factors for glaucomatous optic nerve damage [3]. Low ocular perfusion pressure results in optic nerve blood flow impairment and microcirculatory deficiency which may contribute to glaucoma progression in eyes with either high or normal IOPs [4].
Radial Peripapillary Capillaries (RPCs) are the innermost layer of capillaries that are largely restricted to the posterior pole up to 4-5 mm from the optic disc. The RPCs satisfies the nutritional demands of the inner portion of the RGC around the Optic Nerve Head (ONH) [5]. The unique distribution of RPC makes them vulnerable to IOP rise which can manifest clinically as Bjerrum scotoma [6,7]. The most recent technique for examining vascular changes in the nerve endings is Optical Coherence Tomography Angiography (OCT-A). OCT-A is a noninvasive imaging technique that uses en-face reconstruction of OCT combined with motion contrast to visualize retinal and ONH microvasculature [8]. OCT-A with Split-Spectrum Amplitude-Decorrelation Angiography (SSADA) algorithm reduces scanning time and provides suitable quality because of improved signal-to-noise ratio and minimized motion artifacts [9]. The SSADA algorithm can provide images of perfused capillaries in the perifoveal and peripapillary regions.
Primary Angle Closure Glaucoma (PACG) is a major type of glaucoma with high prevalence [10]. The spectrum for primary angle closure disease manifest from Primary Angle Closure Suspect (PACS), and Primary Angle Closure (PAC) to PACG [11]. Another type of glaucoma, the Pseudoexfoliative Glaucoma (PEXG) is a rapid progressive open angle glaucoma with high baseline IOP and wide IOP fluctuation compared to other types of Primary Open Angle Glaucoma (POAG) [12]. There are some studies suggesting the possible contribution of abnormal microvascular factors in the progression of PEXG [13,14]. The vascular-function relationship between PACG and POAG is different, and the ocular blood flow abnormality in PACG has a lower prevalence compared with POAG [15,16].
Previous studies have reported decreased ONH vessel density in eyes with primary open angle and PEXG [17-20]. To the best of our knowledge, few studies have investigated the circumpapillary and macular vessel density in PACG eyes and eyes which are Primary Angle Closure Suspects (PACS). Insights into the status of the retinal microvasculature could be an excellent indicator of the presence or potential for disease. In the present study we aimed to evaluate the RPC using OCT-A in primary angle closure suspects vs primary angle closure glaucoma vs PEX glaucoma and to compare the results with normal healthy eyes and to investigate correlations between ONH and retinal vessel density measurements to other structural parameters like RNFL thickness. The results of this study may be of help in suspected patients in order to be able to treat patients at risk promptly and consequently reduce the irreversible effects.
All patients in this prospective cross-sectional study were recruited from January 2020 to December 2021. The study protocol was approved by Artesh University of Medical Sciences (AJA) Institutional Review Board and adheres to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all subjects.
All patients underwent complete ophthalmic examination, including Best Corrected Visual Acuity (BCVA), slit-lamp biomicroscopy, gonioscopy, dilated fundus exam and IOP measurement by Goldmann application tonometry.
The study was conducted on four groups of patients. Inclusion criteria for Group A which consisted of Primary Angle Closure Suspects (PACS) were narrow angle on examination with the van Herrick technique which was confirmed as angle closure for >180° with no peripheral anterior synechia on gonioscopy (grade 1 based on Schaffer grading system) and IOP <21 mm Hg without glaucomatous optic neuropathy. Group B were patients with Primary Angle Closure Glaucoma (PACG). PACG was diagnosed when an eye had a primary angle closure with glaucomatous ONH and VF defects. Patients with PEXG (Group C) were clinically detected XFS in addition to open angles on gonioscopy, characteristic glaucomatous optic disc damage, thinning of the Ganglion Cell Complex (GCC), or circumpapillary Retinal Nerve Fiber Layer (RNFL). The control (Group D) consisted of age matched control subjects with healthy eyes and open anterior chamber angles on van Herrick and gonioscopy. The inclusion criteria for the control group were age of at least 18 years, Best Corrected Visual Acuity (BCVA) greater than or equal to 20/30, no evidence of retinal pathology or glaucoma, IOP of 21 mm Hg or less, any chronic ocular or systemic corticosteroid use and normal optic discs on fundoscopic. In all groups patients were excluded if there was history of acute angle closure episode, eye trauma, uveitis and any laser or intraocular surgery (e.g., Laser Peripheral Iridology [LPI]) in either affected or contralateral eyes and patients with high myopia or high hypermetropia (mean diopter ≤ ± 6D). To avoid any inter-eye symmetry, only one eye of each patient was entered in the study.
Ultrasonographic pachymetry (CCT), RNFL-OCT, Ganglion Cell Complex (GCC) and OCTA of the microvasculature in the optic nerve head and macula was performed in all patients. Optical coherence tomography RNFL thickness analysis was performed prior to OCT-A. The average circumpapillary RNFL thickness and eight RNFL sectors were measured at 3.45 mm diameter circle around the optic disc, and defined in clockwise order for the right eye and vice versa for the left eye and designated as Superior Nasal (SN), Superior Temporal (ST), Temporal Upper (TU), Temporal Lower (TL), Nasal Upper (NU), Nasal Lower (NL), Inferior Nasal (IN), and Inferior Temporal (IT). Only good-quality RNFL scans with quality score >20 and continuous scan pattern without motion artifacts and perfect centration were included for analysis. The Ganglion Cell Complex (GCC) includes the RNFL, ganglion cell layer and the inner plexiform layer. The GCC scan (RT-vue 100, SD-OCT) was centered 1 mm temporal to the fovea and covered a 7 mm diameter circular area on the central macula. The average, superior and inferior GCC thickness was recorded for each patient.
Optic nerve head, peripapillary choroidal and macular vessel density were evaluated for each patient using the AngioVue OCT system (Optovue, Inc., Fremont, CA, USA). This system uses an 840- nm light source and has an A-scan rate of 70000 scans/s and a bandwidth of 50 nm. The Split Spectrum Amplitude Decorrelation Angiography (SSADA) algorithm was used to capture the dynamic motion of the red blood cells. Vessel density was calculated automatically as the proportion of measured area occupied by flowing blood vessels defined as pixels having decorrelation values acquired by the SSADA algorithm above the threshold level. Vessel density in the peripapillary RNFL was assessed within a 4.5 × 4.5 mm field of view centered on the ONH. Vessel density within the RNFL was measured from the Internal Limiting Membrane (ILM) to the RNFL posterior boundary using standard AngioVue software, and circumpapillary vessel density were obtained over the entire 4.5 × 4.5 mm2 scan. Macular superficial vessel density measurements were calculated in a slab from the ILM to the posterior border of the inner plexiform layer. Macular whole enface image Vessel Density (wi-VD) measurements were calculated from the entire field of 3 × 3 mm2 scans centered on the fovea.
Poor quality scans, defined as images with (1) a signal strength index of less than 45; (2) residual motion artifacts visible as an irregular vessel pattern or disc boundary on the en-face angiogram; (3) segmentation errors; or (4) a local weak signal caused by media opacities, were excluded.
All statistical analyses were performed by SPSS (IBM Corp, IBM SPSS Statistics for Windows, and Version 25.0. Armonk, NY: IBM Corp.). To present data we used mean, standard deviation, frequency, percentage and range. To compare outcomes between groups one-way Analysis of Variance (ANOVA) with Tukey HSD post hoc test was used. Spearman’s correlation analysis was used to describe the relations between RNFL thickness, wi-VD, cp-VD and GCC. Correlations between macular wi-VD and RNFL thickness were calculated by linear regression analysis. P-value less than 0.05 considered statistically significant.
The study population consisted of 24 (10 men and 14 women) primary angle closure suspect eyes (Group A), 25 (13 men and 12 women) primary angle closure glaucoma eyes (Group B), 20 (12 men and 8 women) PEX glaucoma eyes (Group C) and 30 (19 men and 11 women) healthy eyes for control (Group D). The average age of subjects (range) of groups A, B, C and D were 58.2 ± 8.1 (49–76) years, 59.0 ± 5.4 (50–67) years, 62.4 ± 7.4 (50–77) years and 57.6 ± 7.3 (42–67) years, respectively (p=0.120). The average Intraocular Pressure (IOP) of eyes (range) in Group A, B, C and D were 16.8 ± 2.4 (11–20) mmHg, 18.7 ± 3.6 (12–26) mmHg, 20.4 ± 8.06 (11–43) mmHg and 14.3 ± 3.7 (10–21) mmHg, respectively. Table 1 shows the demographic and baseline characteristics of the study groups.
| Characteristic | Group A (PACS) | Group B (PACG) | Group C (PEXG) | Group D (control) | P value |
|---|---|---|---|---|---|
| Number of eyes | 24 | 25 | 20 | 30 | - |
| Age (years) | 58.2 ± 8.1 | 59.0 ± 5.4 | 62.4 ± 7.4 | 57.6 ± 7.3 | 0.12 |
| Sex F/M | 14/10 | 12/13 | 08/12 | 11/19 | 0.24 |
| IOP (mmHg) | 16.8 ± 2.4 | 18.9 ± 3.6 | 20.4 ± 8.06 | 14.3 ± 3.7 | < 0.001 |
| UCVA (decimal) | 0.7 ± 0.1 | 0.6 ± 0.2 | 0.4 ± 0.2 | 0.7 ± 0.2 | < 0.001 |
Continues data are shown as mean ± standard deviation. Categorical variables were compared using the chi-square test. Continues parameters were compared with one-way ANOVA.
Table 1: Demographic and baseline characteristics of study groups.
RNFL thickness comparisons
The average RNFL thickness in Group A was 106.7 ± 9.4 μm, for Group B was 87.7 ± 12.6 μm, for Group C it was 87.4 ± 16.9 μm and Group D was 113.3 ± 8.5 μm. The NFL thickness in each eight sectors for eyes in Groups A, B, C and D are depicted in Table 2. The NFL thickness in the PACS group (Group A) was statistically similar to the control group (group D), except for the Temporal Upper (TU) section which was thinner in the PACS group (p=0.014). The NFL thickness values in all sectors were significantly lower in Groups B and C compared with the normal control eyes in Group D, except for the Superior Temporal (ST) and the Inferior Temporal (IT) sectors which was only significantly lower in the PACG group (Group B).
| Variables | PACS (Group A) | PACG (Group B) | PEXG (Group C) | Control (Group D) | P value A vs. B | P value B vs. C | P value A vs. D | P value B vs. D | P value C vs. D |
|---|---|---|---|---|---|---|---|---|---|
| RNFL Thickness (µm) | |||||||||
| Global | 106.7 ± 9.4 | 87.7 ±12.7 | 87.4 ± 16.8 | 113.2 ± 8.5 | 0.001 | 0.995 | 0.19 | 0.001 | 0.001 |
| Superior temporal | 129.2 ± 6.4 | 99.0 ± 20.5 | 111.2 ± 27.2 | 119.3 ± 11.6 | 0.001 | 0.107 | 0.17 | 0.001 | 0.335 |
| Temporal upper | 104.2 ± 15.8 | 100.4 ± 18.2 | 72.8 ± 21.6 | 118.2 ± 11.3 | 0.854 | 0.001 | 0.014 | 0.001 | 0.001 |
| Temporal lower | 107.8 ± 17.6 | 73.0 ± 19.9 | 65.4 ± 10.3 | 95.9 ± 25.1 | 0.001 | 0.432 | 0.134 | 0.001 | 0.001 |
| Inferior temporal | 115.6 ± 27.4 | 87.0 ± 14.4 | 110.7 ± 29.0 | 102.0 ± 21.5 | 0.001 | 0.002 | 0.231 | 0.035 | 0.357 |
| Inferior nasal | 111.6 ± 20.4 | 101.5 ± 20.9 | 100.1 ± 22.0 | 116.1 ± 13.6 | 0.153 | 0.969 | 0.831 | 0.014 | 0.012 |
| Nasal lower | 105.6 ± 26.6 | 97.6 ± 16.9 | 67.8 ± 10.5 | 109.6 ± 17.7 | 0.462 | 0.001 | 0.874 | 0.018 | 0.001 |
| Nasal upper | 107.5 ± 25.1 | 89.6 ± 20.7 | 74.5 ± 17.4 | 111.6 ± 17.6 | 0.014 | 0.023 | 0.886 | 0.001 | 0.001 |
| Superior nasal | 113.1 ± 14.6 | 88.3 ± 17.5 | 94.1 ± 12.7 | 112.1 ± 11.9 | 0.001 | 0.593 | 0.997 | 0.001 | 0.006 |
| GCC Thickness (µm) | 112.1 ± 17.7 | 95.1 ± 18.7 | 84.5 ± 13.7 | 118.2 ± 15.6 | 0.009 | 0.083 | 0.613 | 0.001 | 0.001 |
| Superior | 110.9 ± 18.6 | 93.9 ± 19.7 | 84.3 ± 15.6 | 116.7 ± 15.6 | 0.009 | 0.147 | 0.654 | 0.001 | 0.001 |
| Inferior | 115.6 ± 16.9 | 92.3 ± 19.9 | 84.5 ± 14.3 | 115.7 ± 16.3 | 0.001 | 0.287 | 1 | 0.001 | 0.001 |
| CP-VD (%) | |||||||||
| Global | 52.5 ± 2.5 | 42.8 ± 9.3 | 42.7 ± 9.2 | 52.8 ± 2.7 | 0.001 | 0.998 | 0.996 | 0.001 | 0.001 |
| Superior temporal | 54.5 ± 4.2 | 42.5 ± 13.0 | 40.9 ± 14.4 | 54.7 ± 3.9 | 0.001 | 0.877 | 1 | 0.001 | 0.001 |
| Temporal upper | 52.8 ± 4.2 | 50.5 ± 7.9 | 49.9 ± 8.8 | 56.7 ± 3.1 | 0.539 | 0.961 | 0.11 | 0.003 | 0.003 |
| Temporal lower | 53.2 ± 3.5 | 48.3 ± 7.1 | 48.7 ± 5.7 | 54.1 ± 3.7 | 0.008 | 0.972 | 0.904 | 0.001 | 0.004 |
| Inferior temporal | 54.6 ± 3.9 | 43.8 ± 12.1 | 42.9 ± 14.4 | 56.5 ± 4.4 | 0.001 | 0.962 | 0.876 | 0.001 | 0.001 |
| Inferior nasal | 50.2 ± 4.1 | 39.3 ± 11.7 | 40.5 ± 11.7 | 50.7 ± 3.9 | 0.001 | 0.912 | 0.998 | 0.001 | 0.001 |
| Nasal lower | 49.0 ± 4.1 | 39.4 ± 10.2 | 39.8 ± 10.1 | 49.7 ± 4.1 | 0.001 | 0.984 | 0.986 | 0.001 | 0.001 |
| Nasal upper | 50.2 ± 2.7 | 40.8 ± 9.1 | 40.5 ± 9.4 | 50.7 ± 3.1 | 0.001 | 0.989 | 0.993 | 0.001 | 0.001 |
| Superior nasal | 49.9 ± 4.5 | 40.0 ± 11.3 | 39.5 ± 12.8 | 50.0 ± 4.6 | 0.001 | 0.988 | 1 | 0.001 | 0.001 |
Data are shown as mean ± standard deviation.
Parameters were compared with post hoc Tukey’s HSD test.
Table 2: RNFL, GCC Thickness and per papillary vessel density measurements in the four study groups.
GCC analysis comparisons
The macular ganglion cell (GCC) thickness values were thinner in the PEXG group (Group C) compared with Groups A, B and D. GCC values in the PACG (Group B) were thinner than the PACS (Group A) and control group (Group D) (Table 2). Compared with the control group, the inferior and superior hemi-sector GCC thickness were thinner in the PACG and PEXG groups (p <0.001 and p <0.001 respectively). The PACS and control group showed similar GCC thickness statistically.
Peripapillary and macular vessel density
For vessel density in the per papillary area, the sector values in PACS eyes and the control did not show any significant difference. VD in group B and C were significantly lower than those in the PACS (group A) and healthy eyes groups (group D) (42.8 ± 9.3% and 42.7 ± 9.3% vs. 52.5 ± 2.5% and 52.8 ± 2.6%, respectively; P< 0.001). Regarding vessel density in the macular region, the wi-VD values in PACS eye (group A) were statistically similar the control eyes (group D) (409.8 ± 2.4% vs. 50.6 ± 2.7%, P=0.957). Macular wi-VD values in group B were lower than those in healthy eyes (40.9 ± 8.0% vs. 50.6 ± 2.7%, P<0.001) and group C was also lower than those in healthy eyes (41.05 ± 8.0% vs. 50.6 ± 2.7%, P<0.001). (Table 3) illustrates the density of per papillary capillaries evaluated in the 8 peripapillary regions. The absolute values of the differences between superior-hemi and inferior-hemi parafoveal vessel density were relatively greater in PACG eyes than in PEXG and healthy eyes (0.7 ± 3.9 % vs. 0.07 ± 5.1% and 0.35 ± 2.2, respectively), but this difference did not reach statistical significance (P=0.613, Table 2).
| Peripapillary Sectors | PACG (Group B) | PEXG (Group C) | Control (Group D) | |||
|---|---|---|---|---|---|---|
| R | p-value | r | p-value | r | p-value | |
| Superior Temporal (ST) | 0.729 | 0 | 0.656 | 0.002 | 0.076 | 0.689 |
| Temporal Upper (TU) | 0.656 | 0 | 0.642 | 0.003 | 0.349 | 0.059 |
| Temporal Lower (TL) | 0.382 | 0.059 | 0.256 | 0.291 | 0.132 | 0.486 |
| Inferior Temporal (IT) | 0.301 | 0.143 | 0.684 | 0.001 | -0.397 | 0.03 |
| Inferior Nasal (IN) | 0.618 | 0.001 | 0.685 | 0.001 | 0.136 | 0.475 |
| Nasal Lower (NL) | 0.378 | 0.063 | 0.68 | 0.001 | -0.074 | 0.697 |
| Nasal Upper (NU) | 0.231 | 0.267 | 0.613 | 0.004 | 0.16 | 0.399 |
| Superior Nasal (SN) | 0.728 | 0 | 0.71 | 0 | 0.199 | 0.293 |
*Statistical significance tested by the Spearman r correlation.
Table 3: Sector-wise Correlation between Retinal Nerve Fiber Layer (RNFL) Thickness and Sectoral Vessel Density (VD)(Correlation coefficient r).
Correlations between peripapillary vessel density, wi-VD, GCC and RNFL thickness
There was significant positive correlation between VD and corresponding RNFL sector analysis in PACG eyes for the ST (P<0.001), TU (P<0.001), IN (P=0.001) and SN (P<0.001) sectors (Table 3). For the PEXG eyes all sectors except the TL (P=0.291) sector were significantly correlated. There was no significant correlation between VD and corresponding RNFL sector analysis in the control group except for IT sector which showed significant negative correlation (P=0.030) (Table 3). A graph correlating the circumpapillary wi-VD values with average RNFL thickness in each group is shown in Figure 1.
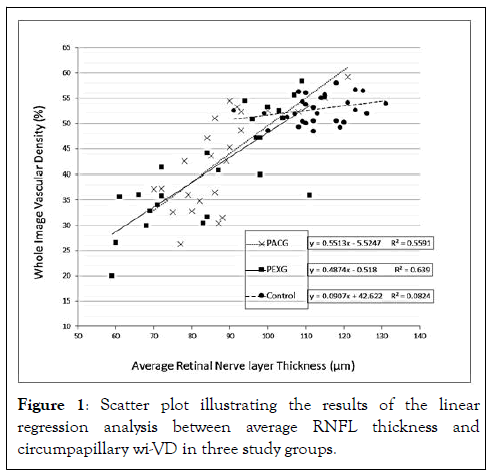
Figure 1: Scatter plot illustrating the results of the linear regression analysis between average RNFL thickness and circumpapillary wi-VD in three study groups.
There was significant correlation between macular whole image VD (wi-VD) and GCC thickness analysis in PACG eyes (Group B) (r=0.407, p=0.043) and PEXG (Group C) (r=0.799, p=0.001). No significant correlation was seen in the PACS group (r= -0.040, p = 0.851) in this regard and control group (r=-0.044, p=0.819) in this regard.
RPC is a layer of retinal vascular network located in the inner surface of the NFL which is involved in the vascular nutrition of ganglion cell axons. This vascular plexus is prone to vasogenic changes and structural changes to the network have been apparent in the pathogenesis of several diseases [21]. Recent studies have demonstrated the morphologic and quantitative characteristics of RPCs on OCT-A, and has shown comparable to the histologic representation and useful in diagnosis and prognosis [22,23] showed that vascular parameters, including vascular density and the distance between large vessels, altered significantly in glaucoma eyes, and the vascular parameters had a high differential ability compared to structural parameters [24]. Suggested that quantitative study of the microvascular perfusion could indicate changes of optic nerve blood flow in the early stages of glaucoma [25]. They showed that in the early stages of glaucoma the optic disc has decreased perfusion which can be detected with OCT-A with 100% sensitivity and specificity [26]. A study on RPC in glaucoma patients shows that the density of this layer is significantly lower in glaucoma eyes than in normal individuals and is also correlated to the degree of visual field impairment and the RNFL thickness [27].
In a large study, showed that the age adjusted vascular density of the optic disc was significantly lower in patients with open-angle glaucoma than glaucoma suspects and normal individuals [28]. Indicated that vessel density using OCT-A was significantly lower in areas of visual field defects in eyes with POAG compared to control [29].
In the current study, we evaluated the quantitative characteristics of Radial Peripapillary Capillaries (RPC) and macular capillaries in Primary Angle Closure Suspects (PACS), Primary Angle Closure Glaucoma (PACG), Pseudoexfolation Glaucoma (PEXG) and normal control eyes using OCT-A. Our results did not show any significant difference in terms of macular Whole Image (wi-VD) and peripapillary (cp-VD) Vascular Density between PACS and control eyes. Two recent studies on PACS eyes also showed similar vessel densities in the peri-papillary region compared to control eyes [30]. However, Pan et al. results indicated that superficial and deep macular VD showed significant decrease in PACS eyes [31]. On the other hand did not find any significant difference between PACS and control subjects in terms of superficial and deep macula vessel densities [32]. Yi Zha et al. also indicated significantly thicker average RNFL in PACS eyes compared to control, whilst other studies [33] similar to our study did not show any difference in this regard. Although when we compared the RNFL thickness sector-wise, we found significantly lower thickness in the temporal upper sector of PACS eyes. Reason for these conflicting results in studies may be different Axial Length (AL) of eyes. Overall, it seems that OCTA parameters and RNFL thickness do not have a diagnostic value for PACS and PACS remains a clinical diagnosis. Conducted a study on 29 eyes with primary angle closure suspect, 22 eyes with Primary Angle Closure (PAC), and 25 eyes with Primary Angle Closure Glaucoma (PACG) and 27 control eyes [34]. They indicated that PAC eyes had lower peripapillary VD than normal eyes while RNFL and GCC thickness did not differ. This finding may verify the effect of IOP on the peripapillary vessel density.
Most previous studies have investigated vascular density in primary open-angle glaucoma and the number of studies on primary angle-closure remains limited. Sihota et al. studied the optic nerve head perfusion in POAG and PACG patients using fluoresceine angiography [35]. They showed that both POAG and PACG patients have delayed choroidal and disc filling, there was a diffuse delay in POAG and a sectoral delay (infratemporal and supratemporal) in PACG eyes. Also described significantly lower vessel density of PACG and POAG eyes in the whole image of optic disc, peripapillary sectors, and parafoveal quadrants using OCT-A [36]. In contrast to, their study showed a reduction in the infratemporal per papillary in POAG eyes while PACG eyes showed a more evenly distributed loss. These studies indicate a structural difference in VD and underlying mechanisms of different glaucoma subtypes.
Our results showed that the macular whole image (wi-VD) and peripapillary (cp-VD) vascular density were significantly reduced in PACG and PEXG eyes compared with PACS and control eyes. The results were similar to previous reports in glaucoma eyes that showed impaired microvasculature in the optic disc and peripapillary region [37,38]. Pseudoexfoliation syndrome is the most common recognizable cause of open-angle glaucoma worldwide. The ONH blood flow can be affected by the accumulation of pseudo exfoliation material, especially in the posterior ciliary arteries. Impaired ocular hemodynamics have been identified using Doppler imaging in pseudo exfoliation syndrome and glaucoma, therefore peripapillary vessel density variations could also occur [39-41]. Found a significant decrease in the wi-VD in PEXG and POAG eyes compared to control; however, no significant difference was seen in the peripapillary VD between patients with POAG and PEXG eyes [42].
To our knowledge no previous reports have compared the sectorial VD change in the optic disc or macula between PACS, PACG, PEXG eyes and healthy subjects. We also compared the RNFL thickness and corresponding optic disc VD in each sector between PACG and PEXG eyes.
Both PACG and PEXG patients demonstrated significantly decreased RNFL thickness values in all sectors compared to normal patients, except for the superior temporal and inferior temporal sector in PEXG patients. RNFL thickness varied significantly in 4 out of 8 sectors between the PACG and PEXG eyes. We also see a significant difference in CP-VD values in the PACG and PEXG groups compared to control eyes, however no difference was observed in sectorial CP-VD values between PACG and PEXG eyes. Both PEXG and PACG eyes showed a significant reduction of circumpapillary VD. Moreover, we did not find any difference between PEXG and PACG eyes in the superior and inferior-hemi GCC thickness. The sectorial RNFL thickness and VD showed significant correlation in the PEXG eyes except for the temporal lower sector. While this correlation was significant for only 4 out of 8 sectors in the PACG eyes.
Previous studies demonstrated that the inferior temporal per papillary VD has the strongest association with the corresponding RNFL thickness and visual sensitivity loss in POAG eyes [43,44]. In contrast noticed an evenly distributed loss in the peripapillary VD. According to our results this matter does not hold true in PACG eyes and the superior temporal and superior nasal had the strongest correlation. As for the PEXG eyes, in consecutive order the superior nasal, inferior nasal and inferior temporal had the strongest association.
The limitation of the present study is the relatively limited sample size. Second, as high refractive error eyes were excluded the results cannot be extrapolated to these patients. Third, blood pressure and underlying systematic diseases such as diabetes were not taken into account for analysis. Furthermore, as this was a cross-sectional study, it cannot be determined whether the RPC’s VD loss precedes the RNFL loss or that is a consequence of raised IOP and RNFL damage.
To conclude, we did not find any differences in VD between control and PACS eyes in peri-papillary and macular region using OCTA. This suggests that other factors such as increases in IOP have an impact on vessel density. However, the OCTA vessel density was significantly reduced in PACG and PEXG eyes in all circumpapillary sectors and the macula. The wi-VD and cp- VD perform as well as the RNFL thickness for discriminating between healthy and glaucoma. This noninvasive method of RPC vessel density assessment is reliable for detecting glaucoma similar to OCT RNFL thickness. Also, OCTA could be of use for monitoring decrease in RPC VD before glaucomatous optic neuropathy and structural changes have occurred in PAC and PEX eyes.
Citation: Shahraki K, Moravvej Z, Shahraki K, Khodaparast M, Makateb A, Shahraki K (2024) A Comparison of Retinal Microvascular Density using OCTA in Primary Angle Closure Suspects, Primary Angle Closure Glaucoma, Pseudo Exfoliation Glaucoma and Healthy Controls. J Clin Exp Ophthalmol. 15:990.
Received: 25-Apr-2022, Manuscript No. JCEO-22-17138; Editor assigned: 27-Apr-2022, Pre QC No. JCEO-22-17138(PQ); Reviewed: 11-May-2022, QC No. JCEO-22-17138; Revised: 27-Jun-2022, Manuscript No. JCEO-22-17138(R); Published: 26-Sep-2024 , DOI: DOI: 10.35248/2155-9570.24.15.990
Copyright: © 2024 Shahraki K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrtribution, and reproduction in any medium, provided the original author and source are credited.