Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Review Article - (2021)
Meibomian Gland Dysfunction (MGD) is the leading cause of Dry Eye Disease (DED). In MGD, obstructed meibomian glands result in reduced meibum secretion and a compromised tear lipid layer that causes tear film instability and an accelerated evaporation of tears. This accelerated and excessive tear evaporation in turn leads to the signs and symptoms of DED. Research has demonstrated that an elevated and sustained therapeutic temperature of at least 41°C at the tarsal conjunctiva located at inner surface of the eyelid can liquefy hardened or thickened meibum and help clear gland obstructions in MGD. By clearing the obstructions, restored meibomian glands can resume the production of meibum that can flow naturally out of the glands and onto the tear surface thereby restoring a stable and healthy tear film lipid layer. Fortifying the lipid layer by enhancing natural meibum production is an effective treatment for evaporative dry eye disease. In recent years, several devices have been developed that utilize thermal energy to treat DED and MGD that require heating from inside the eyelids. The medical community has debated the ability to achieve therapeutic level of temperatures at the tarsal conjunctiva via a non-invasive external approach. This article discusses a new device, TearCare, that has achieved and maintained the requisite 41°C therapeutically-effective, meibum-melting temperature at the tarsal conjunctiva non-invasively through a combination of novel features including: wearability, total tarsal conformance, blink assistance, and software sensor-controlled thermal maximization and optimization.
Meibomian gland disfunction; Thermal therapy; TearCare; Dry eye disease
DED: Dry Eye Disease; FTIR: Fourier Transform Infrared Spectroscopy; MGD: Meibomian Gland Dysfunction; MGSS: Meibomian Gland Secretion Score; OSDI: Ocular Surface Disease Index; TBUT: Tear Break-Up Time
MGD is a leading cause of DED
Strongly associated with dry eye disease (DED), meibomian gland dysfunction (MGD) is a chronic, multifactorial abnormality of the meibomian glands that alters gland morphology and physiology [1]. The most common cause of MGD is the obstruction of meibomian glands that is associated with alterations in meibum chemistry and gland hyperkeratinization [1-3]. Such obstruction leads to hyposecretion and ultimately accumulation of meibum within the meibomian glands [3]. The gland obstruction, if not treated effectively and efficiently, can cause up-regulation of neighboring glands, gland inflammation, atrophy, and dropout. In recent years MGD has been recognized as a common disorder, with a prevalence up to 50% that increases with age [4]. MGD greatly affects the ocular surface leading to tear film instability, rapid tear evaporation and drying, tear hyperosmolarity, and subsequent inflammatory damage of the ocular surface. Clinically, these changes result in symptoms such as visual degradation, blurred vision, ocular fatigue, ocular discomfort, and foreign body sensation. In other words, obstruction of the meibomian glands leads to DED (Figure 1) [3].
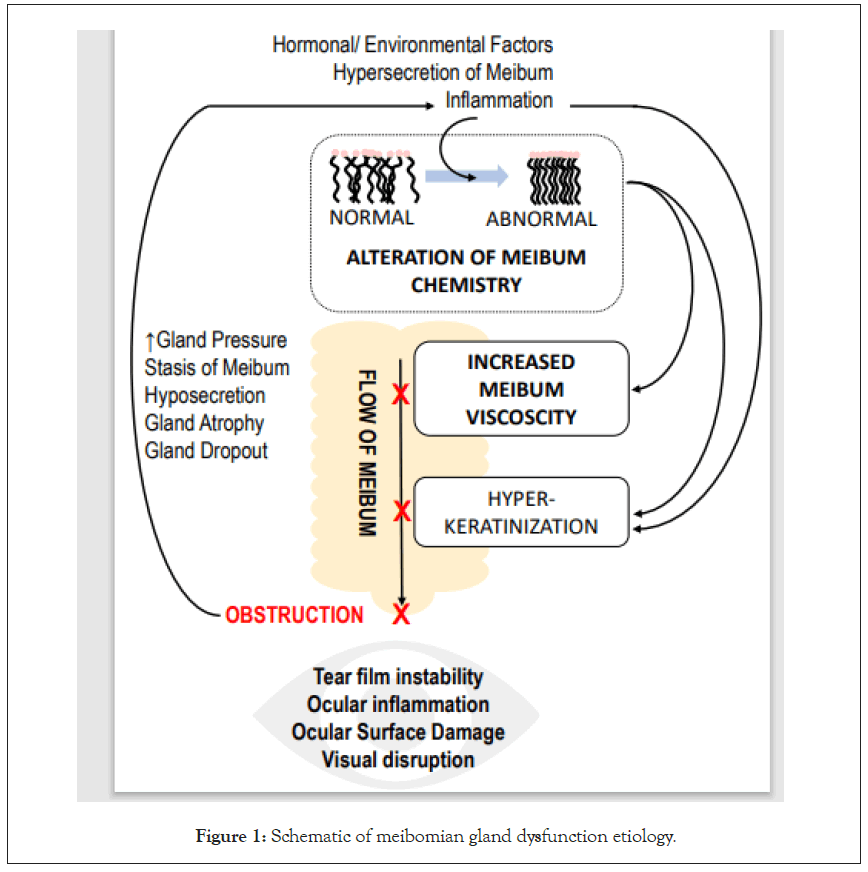
Figure 1: Schematic of meibomian gland dysfunction etiology.
DED is the most frequently diagnosed ocular morbidity and is defined as “a multi-factorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles” [5]. It is clear from its definition that DED and MGD share commonality in etiology underpinned by tear film instability, ocular inflammation, visual disruption, and ocular surface damage. Fundamentally, DED can be caused by either inadequate tear production or excessive tear evaporation. It has been posited that instability of the lipid layer resulting from MGD is the underlying cause of excessive tear evaporation in DED (Figure 2) [6]. Specifically, MGD is reportedly the leading cause of DED with more than 86% of patients with DED in the US found to have underlying MGD [3,7]. In these cases, it is likely that MGD is responsible for many of the hallmarks of DED. With this in mind, great efforts have been taken to reduce the obstructions associated with MGD to improve tear film stability and to reduce evaporation.
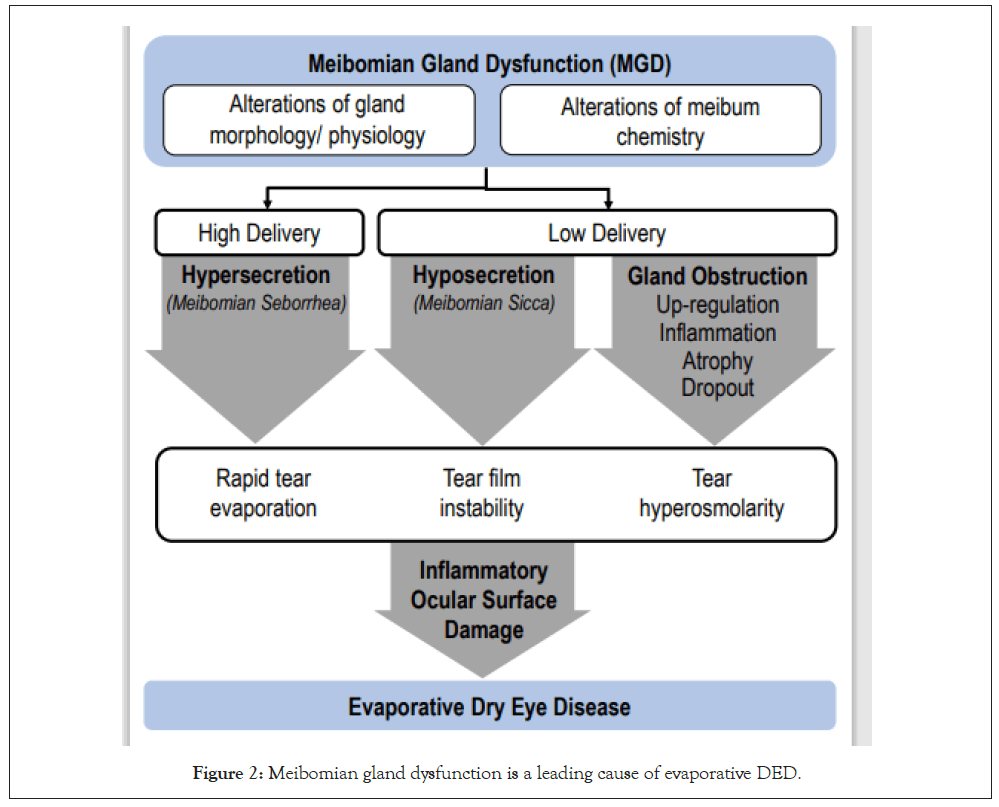
Figure 2: Meibomian gland dysfunction is a leading cause of evaporative DED.
DED is a significant public health issue as it has significant negative impact on the visual function, quality of life and productivity of patients, and is highly prevalent in the working age population and above. Approximately one-third of patients visiting their eye doctor suffer from dry eye. The prevalence of dry eye disease increases with age, especially in postmenopausal women. It is estimated that dry eye disease affects more than 7 million Americans older 40 years of age, and approximately 1 million to 4 million Americans between 65 to 84 years of age. [1,3,7] In addition to the challenges of daily life faced by sufferers of DED, the compromised ocular surface in DED often affects the outcomes of refractive surgery, cataract surgery, and contact lens use [8].
Recently, a great deal of attention has been directed at evaporative dry eye since it is implicated in the majority of dry eye cases. [3,7]. This is in direct contrast with previous approaches utilizing agents such as topical cyclosporine or artificial tears to treat aqueous insufficiency, which affects the minority of patients with dry eye. Despite this, these approaches are used much more broadly and often ineffectively. There are existing therapeutic strategies for treating evaporative DED; however, there is often a lack of efficacy across the broad DED patient population [9]. As MGD is a major contributing factor in DED, restoring natural meibum secretion and meibomian gland function has been an increasingly popular therapeutic strategy. Thus, alleviating meibomian gland obstruction is an essential step in the treatment of MGD. Currently, the standard of care to manage meibomian gland obstruction is the application of warm compresses. Due to the many limitations of warm compresses, variations of thermal treatments are being developed and gaining popularity amongst practitioners [10]. Over the last decade several devices have been introduced as the first line of treatment for MGD-associated DED by many care givers [11-13]. These devices approach thermal delivery to the meibomian glands in a variety of ways. Herein, we seek to provide a review of meibomian glands and pathophysiology of gland obstruction in the etiology and prognosis of MGD and DED with a focus on the role of sufficient heat as a therapeutic strategy for management of MGD, and therefore, evaporative DED. In addition, this article seeks to discuss the safety and effectiveness of the innovative TearCare system in the treatment of DED. TearCare wearable eyelid technology non-invasively achieves therapeutically-elevated melting temperatures within the meibomian glands while simultaneously preserving the natural blink function to assist in the movement of melted meibum and clearance of gland obstructions.
Meibomian glands, meibum, and the pathophysiology of meibomian gland obstruction
The Meibomian glands, central to MGD and evaporative DED, are large, modified sebaceous glands located in parallel arrangement spanning vertically within the tarsal plates of the eyelids, covered with tarsal conjunctiva as a lining to the inner surface of eyelids. There are approximately 20 to 30 meibomian glands in the lower lid and 30 to 40 in the upper lid and they appear as grape-like clusters attached to a central stalk (Figure 3A) [14]. Each gland has numerous acinar cells connected to a central stalk like ducts lined with keratinized epithelium. The duct orifices are positioned between the lashes and the bulbar conjunctiva. One of the main functions of these glands is to produce an oily substance called meibum which is transported through basal exudation to the fornix through the ductal system (Figure 3B). Meibum forms a superficial lipid layer (0.11 μm) of tear film and its principal function is to spread over the aqueous component of the tear film to retard tear evaporation and assist in the creation of a smooth optical surface [15].
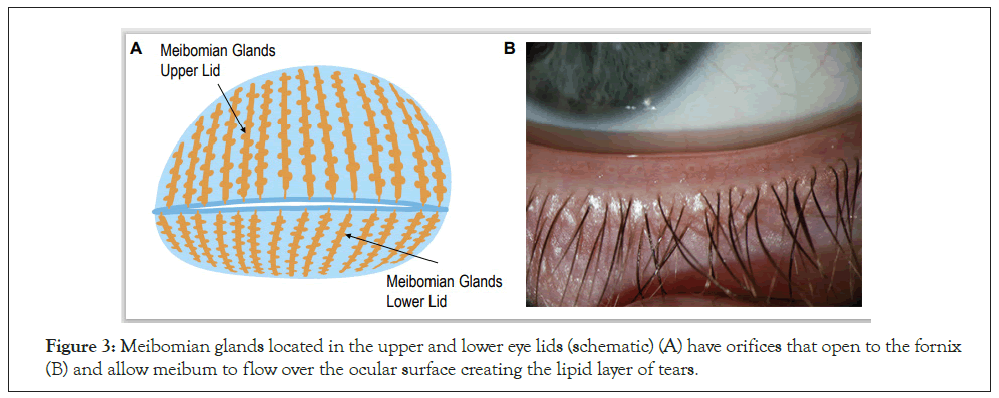
Figure 3: Meibomian glands located in the upper and lower eye lids (schematic) (A) have orifices that open to the fornix (B) and allow meibum to flow over the ocular surface creating the lipid layer of tears.
Meibum is a complex mixture of various polar and nonpolar lipids and contains cholesterol, triacylglycerol, free fatty acids, and phospholipids [15,16]. The melting point of meibum, also known as phase transition temperature, ranges between 19.5°C to 32.9°C in healthy eyes; this temperature range is below the eyelid’s normal surface temperature of 36°C [16]. As a result, healthy eyes meibum is maintained in an oil-like state and is secreted easily from the glands to ultimately make up the lipid layer of tears [2]. The interaction between the lipid layer and the lipophilic proteins of the aqueous layer help to stabilize the tear film and slow down evaporation of the aqueous component [2]. Specifically, the thickness and composition of this lipid layer influences the rate of tear evaporation.
In MGD, dysfunction largely arises from the obstruction of meibomian glands that involves alterations of meibum lipids, hyperkeratinization, blockage of the gland, cystic dilation, and potentially subsequent gland atrophy [17]. Obstruction of meibomian glands in MGD in turn leads to pathological alterations in the critical lipid components of meibum in which the lipids have an abnormally higher number of trans rotamers creating a molecular arrangement that is effectively more ordered as the lipid chains can pack tightly together [18]. Using Fourier transform infrared spectroscopy (FTIR), Borchman et al. have shown that in normal eyes the lipid layer remains approximately 37% ordered, between solid (gel) and liquid (liquid-crystalline) phase at the physiological lid temperature (Figure 4A) [18]. In eyes with MGD, the proportion of ordered meibum was shown to be 30% higher resulting in a 4°C increase in the phase-transition temperature changing it from 30.3°C in normal eyes to 34.0°C in eyes with MGD [19]. This necessitates that a higher temperature be reached in order to transition from the pathological, ordered, more viscous gel-like state, to healthy, disordered, less-viscous oil- like state. The pathological molecular arrangement of lipids seen in MGD makes meibum more viscous thus impeding its natural flow out of the meibomian gland to the tear film and can lead to or exacerbate meibomian gland obstructions. In addition, it does not easily spread in a lipid layer over bulbar conjunctiva (Figure 4B) [6,8,20,21]. Meibum abnormalities in turn cause abnormalities in the lipid layer of the tear film which exacerbates MGD and evaporative DED.
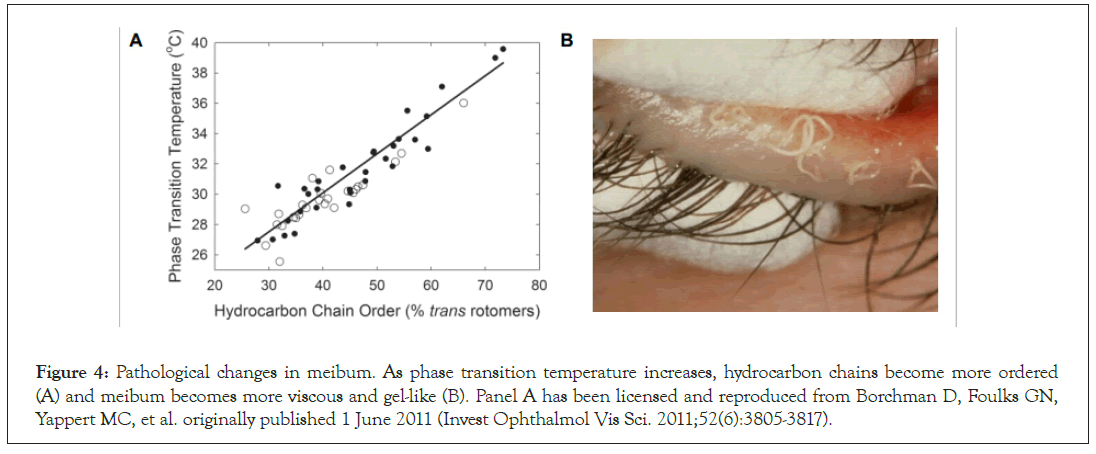
Figure 4: Pathological changes in meibum. As phase transition temperature increases, hydrocarbon chains become more ordered (A) and meibum becomes more viscous and gel-like (B). Panel A has been licensed and reproduced from Borchman D, Foulks GN, Yappert MC, et al. originally published 1 June 2011 (Invest Ophthalmol Vis Sci. 2011;52(6):3805-3817).
Heat application in management of MGD and DED
A stable and normal functioning tear film is the key to maintaining the health of the ocular surface. As such, the first step in treating MGD-related DED is to ameliorate the dysfunctional homeostasis of the tear film, which cannot be achieved without restoration of the lipid layer. Removing the obstruction of meibomian glands is the most essential step in the treatment for MGD-associated DED [10,22].
Lid hygiene, intraductal probing, topical or systemic medications, application of heat, and lid expression have all been employed in the treatment of MGD [10,13]. Debridement alone does not target the deeper gland ducts [10]. Gland expression has also been used, but this can be ineffective in some patients with severe blockages and is often sufficiently uncomfortable for patients that comprehensive gland clearance is difficult [23]. Intraductal probing treatments are more effective in alleviating severely blocked meibomian glands, but this invasive procedure has some limitations with the requirement of topical anesthesia to alleviate pain and the potential for orifice hemorrhaging and gland damage [24].
Another approach to treating MGD involves the use of thermal energy to liquify meibum. Direct application of heat to the meibomian glands potentially avoids many of the pitfalls mentioned above and can work in tandem with the patient’s native physiology to manage MGD and DED. Specific elevated therapeutic temperature requirements have been shown to facilitate conformational changes in meibum lipids which lead to increases in the proportion of the disordered lipid molecules [22]. Greater amounts of disordered lipids enable meibum to transition from a pathologic, gel-like state to a healthy, oil-like state. The heat-induced liquefaction of the abnormal meibum improves delivery of meibum to the ocular surface through the patients native meibum secretion mechanisms, initiated by blinking [18,25,26]. This leads to a healthier, thicker lipid layer that further retards evaporation. The melted meibum is then available to spread over the aqueous component of the tear film during a blink, effectively reducing signs and symptoms of MGD and DED, and ultimately improving patients’ quality of life [27- 30].
The oldest form of heat delivery involves the use of warm compresses, which remains the standard of care in MGD treatment today despite the safety and effectiveness shortcomings. From a safety perspective, when heat is applied to the closed eyelid, it is transferred from the palpebral conjunctiva to the tear film and then to cornea and it is imperative that any thermal treatment minimize heating of the cornea [27]. In addition to warm compresses potentially being overheated and creating risk to the outer skin of eyelid, a non-targeted closed eye heating approach also runs the risk of trapping heat behind the closed eyelids and resulting in overly elevated corneal temperatures. In a warm compress research study, mean corneal temperature climbed to 39.7°C after application of a 45°C warm compress for 8 minutes [31]. The ultimate goal of any thermal MGD therapy should therefore maximize the temperature at the meibomian glands within the eyelids without adversely affecting corneal temperature and health.
From an effectiveness perspective, the challenge with external heat sources, including warm compresses, is whether they can effectively elevate temperatures of the meibomian glands sufficiently to liquify meibum given the highly vascular anatomy of eyelids. Borchman et. al. suggested that the optimal temperature to obtain 90% maximum disorder of meibum lipids for removal of obstruction and clearance of glands in eyes with MGD is 41.5 °C and that this temperature must be maintained for at least several minutes [22]. It was also established that there is a differential of several degrees between temperatures of the inner and outer surfaces of the eyelid. Finally, temperature levels above 45°C are reported to be possibly detrimental to the skin and conjunctiva [32,33]. A single warm compress that is applied to the closed eye, non-targeted in its thermal delivery, not conformed to the tarsal plates and not adhered to the eyelids, and therefore, cannot safely and controllably maintains the elevated therapeutic levels of temperature at the tarsal conjunctiva for a sufficient period of time required for effective MGD treatment. For the reasons above, it is understandable why the “gold standard” warm compress is often ineffective for the treatment of MGD.
A study by Blackie et al. successfully demonstrated that in a research lab setting where external warm compresses were a) applied in a highly stringent and controlled manner and b) replaced every minute with a new warm compress using successive warm compresses, therapeutic temperature targets for the softening and liquefaction of meibum could be achieved [31]. While this proved the possibility of effectively treating the Meibomian glands from an external approach, the regimen of using 10-15 sequential warm compresses on a regular basis is neither practical nor feasible.
As sustained and sufficient therapeutic temperatures at the level of the meibomian glands are critical for the effective clearance of hardened meibum obstructions, optimization of thermal treatments has been sought. The TearCare System (Sight Sciences Inc. Menlo Park, CA) is the first wearable, open-eye eyelid technology for MGD. TearCare leverages highly conformant, sensor-software controlled, blink-assisted, thermally-optimized devices to achieve therapeutically effective temperatures at tarsal conjunctiva to liquefy meibum followed by manual gland expression to expel any residual gland obstructions. The TearCare thermal cycle applies 45°C heat at the outer surface of the eyelid to achieve the optimal temperature of 41°C at the tarsal conjunctiva for 15 minutes to melt obstructions in all meibomian glands underneath the tarsal conjunctiva. The TearCare procedure allows the patient’s eyes remain open and free to blink, resulting in the natural flow of meibum in its melted phase.
Tearcare device and procedure
The TearCare procedure takes place in the monitored setting of a medical professional’s office. The TearCare system consists of a SmartHub controller, charging nest, charging adaptor, and the single use SmartLids that are attached to the upper and lower eyelids of both eyes at the same time. Thermal therapy utilizing the TearCare system is initiated by activating the SmartHub controller and the system automatically and gradually increases the temperature over 2 3 minutes until it reaches the maximum temperature of 45°C which is maintained through 15 minutes. The treatment temperature is optimized and maintained by constantly communicating (240x per second) with the SmartLid sensors on an eyelid-by-eyelid basis to ensure that each eyelid and all meibomian glands are being maximally treated throughout the entire 15-minute thermal cycle. The adherent, disposable SmartLids, that are powered by the SmartHub, utilize a medical grade adhesive on the surface of the SmartLids that physically and thermally couples the devices to the external surface of the eyelids. A SmartLid needs to be affixed to the external surface of each eyelid along the eyelid margin to precisely target the terminal ducts of the meibomian glands and is custom designed to conform to the tarsal plate of each unique eyelid so that patients can blink normally throughout the procedure. The patient is able and encouraged to blink during therapy to naturally express liquefied meibum and better facilitate the manual expression of meibum afterwards (Figure 5).
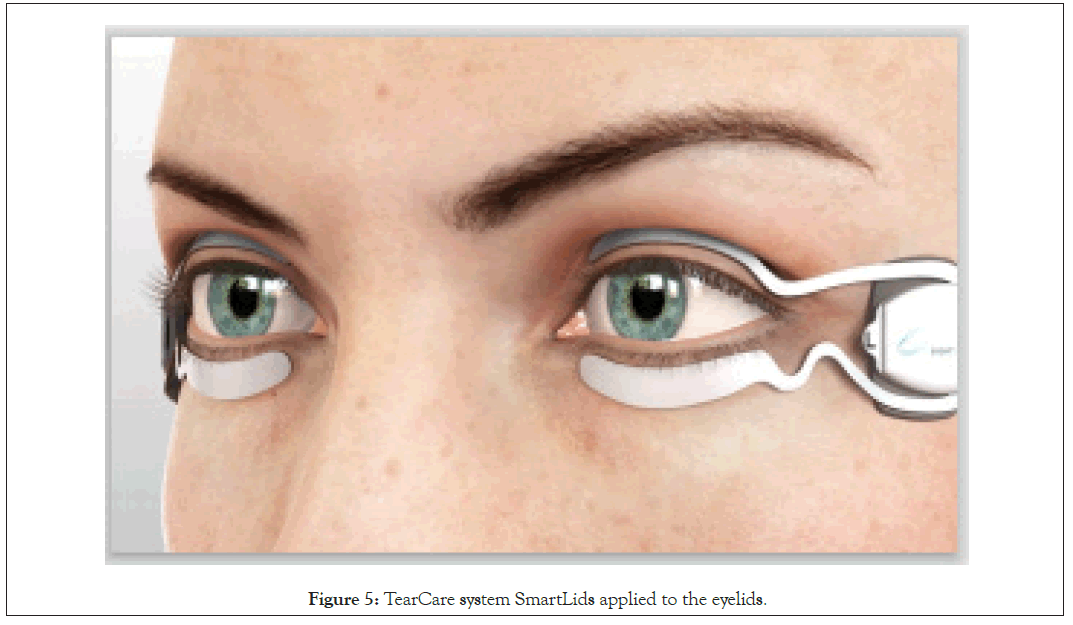
Figure 5: TearCare system SmartLids applied to the eyelids.
Following the eyelid thermal treatment, the clinician uses the TearCare Clearance Assistant to individually express each meibomian gland under direct visualization thereby delivering not only a customized eyelid-by-eyelid treatment but also a personalized gland-by-gland treatment which may allow for a more complete evacuation of liquified meibum [34,35].
TearCare safely and optimally delivers therapeutic heat trans- tarsally
The effectiveness and safety of the initial TearCare system in treating the signs and symptoms of DED was demonstrated previously [36,37]. The safe, controlled delivery of heat to achieve sufficiently elevated temperatures at tarsal conjunctiva was validated in a recent study where 15 subjects were treated with 30 SmartLids with one device per eye. The precise temperature control and heat delivery of the TearCare system was assessed via temperature collection from the cornea, tarsal conjunctiva, and external interface between the eyelid skin and SmartLid (Figure 6A).
During this TearCare validation study, after the SmartHub reached the 45˚C maximum therapeutic temperature and maintained that temperature through 15 minutes, the temperature at outer surface of eyelid had risen an average of 8.8°C (from 35.0 to 43.8°C) while the average temperature of tarsal conjunctiva had risen 6.0°C (from 35.2 to 41.1°C) to the therapeutically effective 41°C meibum melting temperature. The mean corneal temperature had risen only 1.5°C during the TearCare procedure. Post-procedure mean corneal temperature was 36.4°C, while the maximum measured corneal temperature was 37.1°C. Five minutes after the TearCare procedure was completed, tissue temperatures had returned to within 0.6°C of the baseline temperatures (Figure 6B).
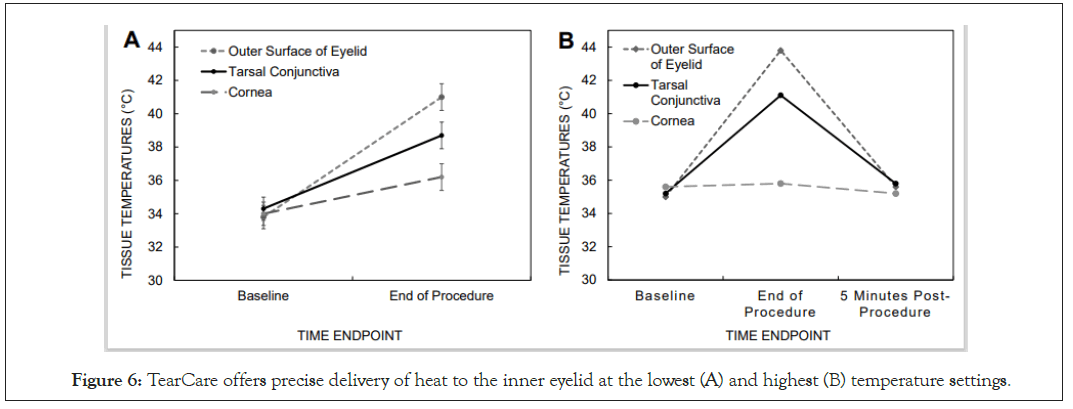
Figure 6: TearCare offers precise delivery of heat to the inner eyelid at the lowest (A) and highest (B) temperature settings.
TearCare was demonstrated to be safe and well-tolerated by patients as evaluated by slit-lamp biomicroscopy, best-spectacle- corrected visual acuity, and corneal and eyelid temperature measurements. During the study no adverse events or clinically significant changes in visual acuity were observed. Notably, during the thermal treatment, the corneal temperature increased by only 1.5°C while a therapeutic temperature increase of 6°C was demonstrated for the tarsal conjunctiva; thereby demonstrating optimization of both safety and efficacy. All temperature increases were well within the safe and tolerable thresholds to not produce any heat-induced ocular damage. Additionally, upon heat removal after the completion of the TearCare thermal procedure, ocular tissues returned to within 0.6°C of the baseline tissue temperatures within 5 minutes of the thermal procedure cessation.
In summary, the TearCare thermal procedure enables safe, optimized, and tightly targeted and controlled temperatures at the tarsal conjunctiva that induce melting phase transitions in meibum lipids from a more ordered gel-like state to a liquid- like oil state that enable meibum to be more easily secreted and cleared from the meibomian glands [16,22].
Tear care is effective in the management of MGD in DED
In a recent multicenter, prospective, single-arm, post-market, exploratory treatment study designed to evaluate safety and effectiveness of the TearCare system against both the signs and symptoms of MGD and DED, 29 subjects (58 eyes) received one TearCare treatment and were assessed at baseline and followed at 1 week and 1 month (ClinicalTrials.gov Identifier: NCT03588624) [36]. The primary effectiveness endpoint was defined as mean change from baseline in Tear Break-up Time (TBUT) in seconds. The consistency of meibomian gland secretions cumulatively for 15 lower-eyelid glands on a Meibomian Gland Secretion Score (MGSS) scale from 0-3 (0=no secretion, 1=toothpaste, 2=cloudy, 3=clear; a higher number indicates more normal meibomian gland function) was assessed as a secondary endpoint along with corneal and conjunctival staining scores and dry eye symptom and quality of life using the Ocular Surface Disease Index (OSDI) questionnaire. At baseline the subject population had DED with the following signs and symptoms: TBUT (seconds)=3.7 ± 1.1; Meibomian Gland Score (cumulative for 15 lower eyelid meibomian glands)=5.6 ± 4.0; Corneal Staining Score=4.8 ± 2.5; Conjunctival Staining Score=5.9 ± 3.2) and symptom (OSDI=54.9 ± 20.2). Following only 1 administration of the TearCare procedure, subjects saw significant improvements in the signs and symptoms of MGD and DED at 1 week that continued at least 1 month after the procedure (Figure 7).
Subjects saw a statistically significant improvement in TBUT of 2.6 seconds (ranging from 2.2 to 6.4 seconds) longer than baseline at 1 week that continued to improve to an average of 3.1 seconds (ranging from 2.6 to 8.3 seconds) longer than baseline at 1 month following the procedure; both p<0.001 (Figure 7A). Similarly, the consistency of meibomian gland secretions was significantly improved from baseline following treatment with the TearCare procedure at all time points; a cumulative score for secretions from 15 lower lid meibomian glands of 14.9 ± 7.0 was observed at 1 week and remained stable at 1 month (cumulative score=14.4 ± 7.3) (Figure 7B). Subjects also saw improvement in mean corneal and conjunctival staining following treatment (Figure 7C). Mean corneal staining was reduced from 4.8 ± 2.5 at baseline to 3.5 ± 2.2 at 1 week and 4.1 ± 2.8 at 1 month post- treatment. Similarly, mean conjunctival staining was reduced from 5.9 ± 3.2 at baseline to 3.4 ± 3.1 and 4.7 ± 3.0 at 1 week and 1 month post-treatment, respectively.
Improvements in DED symptoms were assessed with the OSDI questionnaire to assess ocular symptoms, their impact on patient vision-related functioning, and environmental factors triggering DED symptoms [37]. Following the TearCare procedure, subjects saw clinically and statistically significant improvements in DED severity as scored by OSDI at both 1 week and 1 month following (Figure 7D). Importantly, a majority of subjects (83%) showed clinically meaningful improvements with OSDI score improvements ≥ 13.4 and 66% of subjects saw an improvement in severity from severe DED to moderate DED.
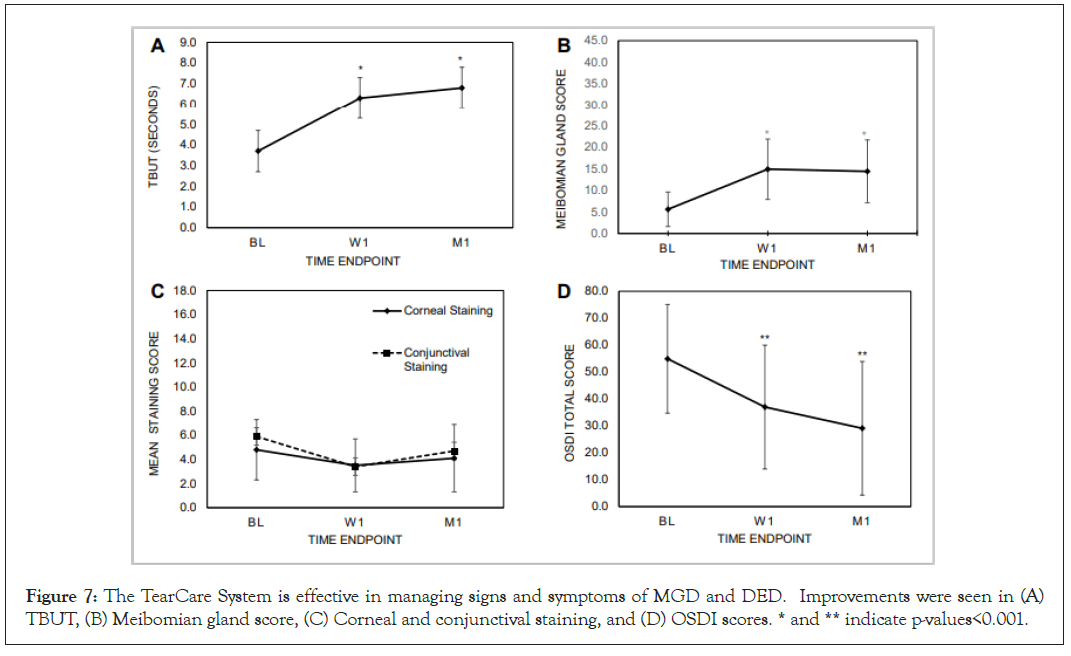
Figure 7: The TearCare System is effective in managing signs and symptoms of MGD and DED. Improvements were seen in (A) TBUT, (B) Meibomian gland score, (C) Corneal and conjunctival staining, and (D) OSDI scores. * and ** indicate p-values<0.001.
A single TearCare treatment resulted in improvements in both the signs (TBUT, MGSS, Corneal and Conjunctival Staining) and symptoms (OSDI) of DED. Additionally, no safety findings or ocular adverse events were reported. From these results, it is evident that the TearCare system, leveraging intelligently optimized trans-tarsal heat transfer, blinking and natural gland clearance, and manual gland expression, is safe, well-tolerated, and effective in the management of MGD and DED.
Despite MGD being the leading cause of DED and affecting 86% of dry eye patients in the US, the majority of DED treatments target pure aqueous deficiency DED, which accounts for a minority of patients with DED. These include the use of artificial tears, which may or may not temporarily relieve symptoms but do not solve the underlying etiology of disease, or costly prescription eyedrops that target aqueous insufficiency (“aqueous deficient dry eye”) but do not address the majority of cases of DED, specifically evaporative DED associated with MGD. Existing dry eye solutions are not without caveats and limitations and have shown a lack of demonstrated efficacy across broad patient populations. There is no prescription drop approved for evaporative DED associated with MGD today.
Managing MGD with medical devices is becoming a preferred method to treat DED. Medical device innovations designed to deliver controlled, thermal therapy to the eyelids to sufficiently liquify hardened meibum and clear gland obstructions have been gaining traction. Important research by Borchman et.al. discovered that the optimal temperature to obtain 90% maximum disorder of meibum lipids in eyes with MGD is 41.5°C. The historical challenge has been to achieve a therapeutically sufficient temperature at the tarsal conjunctiva with a non-invasive externally applied heat source. Achieving the therapeutic temperature level of 41°C at the tarsal conjunctiva requires optimized trans-tarsal deployment of energy for a sufficient period of time to effectively penetrate highly vascular eyelid tissue.
The TearCare System was designed to address this challenge and to deliver optimal therapeutic temperature at the tarsal conjunctiva in the form of a safe, non-invasive, and effective wearable eyelid technology. As evident by the clinical validation data presented earlier, the TearCare System reliably achieves a therapeutically effective temperature of 41°C at the tarsal conjunctiva without the risk of exceeding safety thresholds for the external surface of the eyelid or to the cornea unlike closed eye techniques. The System’s eyelid-worn therapeutic devices, SmartLids, offer conformance and adherence across the entire geography of eyelids to maximize trans-tarsal thermal energy deployment for targeted heat delivery to the underlying meibomian glands. TearCare software and sensor technology enable the tight control and maintenance of a maximized external eyelid temperature of up to 45°C for a sufficient period of time (15 minutes). The TearCare Clearance Assistant permits practitioners to attempt complete removal of liquified meibum with a tailored gland-by-gland approach under optimal visualization as opposed to gland clearance in devices using automated and invisible approaches of gland massaging. Ultimately, this safe and effective clearance of meibomian gland obstructions with TearCare breaks the vicious cycle of evaporative DED associated with MGD.
TearCare is the first and only non-invasive eyelid technology to our knowledge that has achieved the 41°C therapeutic temperature threshold at the tarsal conjunctiva lining the inner surface of eyelid using an externally applied energy source. Through its array of sensor-controlled SmartLids, TearCare is able to continuously deliver a therapeutic level of thermal energy externally that was only possible previously in a research lab setting using labor intensive approaches too impractical for a clinical setting. Verified now through several human validation studies and clinical trials, TearCare’s software controlled, blink-assisted, wearable eyelid technology represents an exciting therapeutic innovation for MGD, the underlying cause of the majority of DED cases.
This study and the validation study were performed under the approval of Aspire Institutional Review Board as a non-significant risk device study. All tenets of the Declaration of Helsinki for the protection of human subjects in medical research were followed in the conduct of these studies.
This work was funded by Sight Sciences, Inc. The authors declare no competing interests in this work.
Citation: Dhamdhere K (2021) A Blink-Assisted, Cornea-Sparing Wearable Eyelid Device for the Effective Penetration of Therapeutic Thermal Energy into the Meibomian Glands for the Treatment of Dry Eye Disease. J Clin Exp Ophthalmol. S12:003.
Received: 11-Jan-2021 Accepted: 25-Jan-2021 Published: 01-Feb-2021 , DOI: 10.35248/2155-9570.21.s12.003
Copyright: © 2021 Dhamdhere K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.