Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Case Report - (2023)Volume 14, Issue 6
Background: We report a case of a symptomatic small Uveal Melanoma (UM) followed for 4 decades, the most common primary intraocular tumor that regressed almost completely after an infectious disease, Tularemia, caused by Francisella tularensis.
Case presentation: In 1981, a 47-year old nurse was diagnosed with a left-sided amelanotic juxtapapillary lesion presumed to be a melanoma. In 1985, the visual acuity decreased to 0.5. The tumor became increasingly pigmented and Sub-Retinal Fluid (SRF) was observed. Ultrasound B-scan demonstrated findings characteristic of a UM. Enucleation was not recommended due to small size and useful vision. The lesion was evaluated annually with fundus images and ultrasound B-scan. In 1993, an epidemic nephropathy presented with anuria. The serum tests, Immunoglobulin M (IgM) and IgG, were positive for Tularemia. Her vision deteriorated to 0.04 in 1995. A central depigmented zone, disappearance of SRF and minimal growth in height were interpreted as signs of regression. Metastatic work-up revealed no pathology. Neither a Fine Needle Aspiration Biopsy (FNAB) nor brachytherapy was recommended.
In 2023, the Best Corrected Visual Acuity (BCVA) was finger counting in her left eye. The area of the previous mass was atrophic except for a small, pigmented area in the upper central corner of the primary lesion, and the lesion was flat on the ultrasound B-scan.
Conclusion: Tularemia most likely initiated the almost complete spontaneous regression of UM.
Uveal neoplasms; Melanoma; Spontaneous neoplasm regression; Tularemia
Uveal Melanoma (UM) arises from the melanocytes in the uveal tract. Although rare, this is the most common primary intraocular tumor in adults with an incidence about 5 per million in United States.
The diagnosis of UM is in contrast to other malignancies still accepted as a clinical diagnosis by oncologists and cancer registries and is mainly based on clinical investigations with indirect ophthalmoscopy slit-lamp biomicroscopy and ultrasound B-scan. The diagnosis of small melanoma is challenging due to its similarity to choroidal nevi [1]. The treatment of UM is highly influenced by the tumor stage with a wide spectrum of modalities of treatment from observation of presumed small melanoma or using an eye saving treatment with irradiation either as local brachytherapy or proton beam. Enucleation is an option if the visual potential is minimal or the probability of local tumor control is uncertain.
Spontaneous regression of malignant diseases is rare, but a documented reality [2]. Both the primary tumor and the metastatic manifestation can disappear. A probable cause of metastatic melanoma of unknown primary is a complete spontaneous regression. In contrast to cutaneous melanoma, this phenomenon is extremely uncommon in UM and then mostly observed as an incomplete or as a transitory event [3]. Such changes are not synonymous with cure and relapse is common. The pathological and gene profiling evidence has been presented only in a few cases [4].
The prognosis of UM is strongly associated with the tumor size at diagnosis. It is documented that even small UM can be fatal [5].
Therefore, the dilemma to observe before initiating treatment is not a simple decision as illustrated in the paper of presumed UM and the dissertation Micrometastasis in UM is the relevance for dormancy [6,7].
Intuitively, we propose that small lesions as our case have a higher probability for spontaneous regression as shown in an old autopsy study of occult papillary carcinoma of thyroid [8].
Tularemia is an infectious disease caused by Francisella tularensis, a gram-negative bacterium [9]. In Europe, the less virulent strain of tularemia, type B subspecies holarctica is more common. In most people, the infection leads to an acute septic reaction with necrosis, microabscesses and has ability to cause significant illness and morbidity. The route of infection varies and therefore also the clinical symptoms.
This case report describes the presentation of a 47-year old woman with a history of tularemia and regression on an untreated juxtapapillary amelanotic choroidal melanoma more than four decades following initial diagnosis.
A 47-year-old Norwegian nurse was initially diagnosed and observed, from 1981 to 1985 at the Eye Clinic of Cornell University Hospital in New York (USA) with a presumed amelanotic juxtapapillary uveal melanoma in her left eye as shown in Figure 1A.
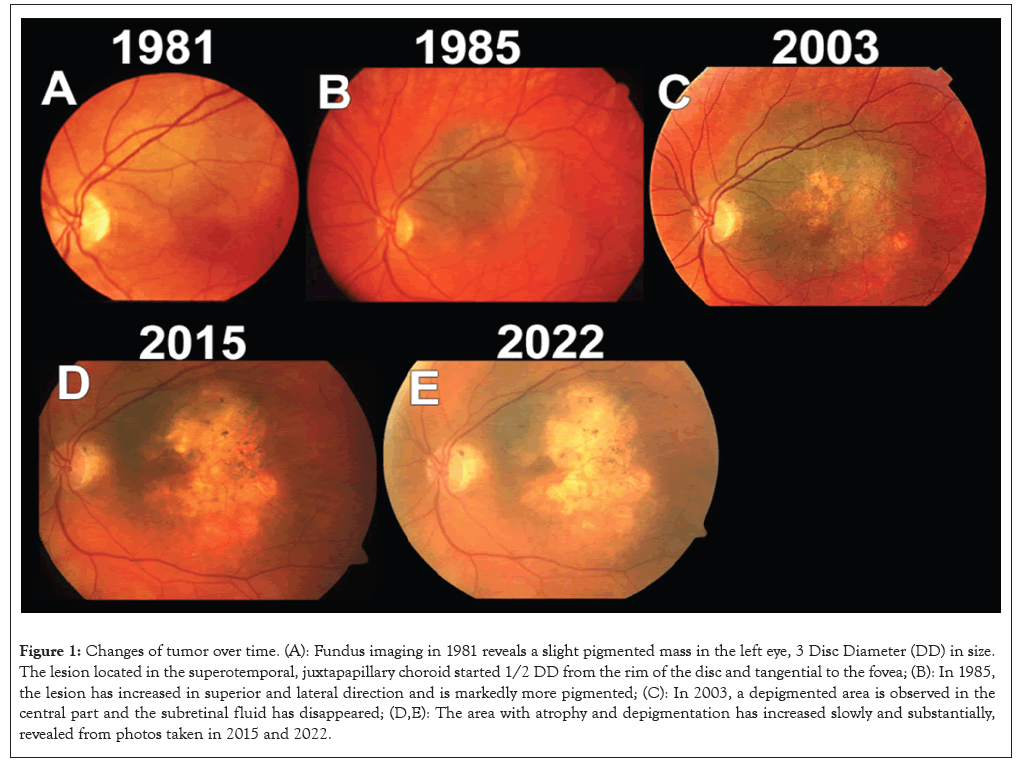
Figure 1: Changes of tumor over time. (A): Fundus imaging in 1981 reveals a slight pigmented mass in the left eye, 3 Disc Diameter (DD) in size. The lesion located in the superotemporal, juxtapapillary choroid started 1/2 DD from the rim of the disc and tangential to the fovea; (B): In 1985, the lesion has increased in superior and lateral direction and is markedly more pigmented; (C): In 2003, a depigmented area is observed in the central part and the subretinal fluid has disappeared; (D,E): The area with atrophy and depigmentation has increased slowly and substantially, revealed from photos taken in 2015 and 2022.
When she returned to Oslo (Norway), her lesion was followed-up at the Department of Ophthalmology, Oslo University Hospital. In January 1985, a lesion was seen originating half a Disc Diameter (DD) from the rim of the optic disc at 3 o’clock position, 4 × 3 DD in size. The moderately pigmented lesion ended at the superior arcade, but did not reach the inferior arcade where the SRF over the lesion ceased as shown in Figure 1B. The BCVA was 1.0 Oculus Dexter (OD) and 0.5 Oculus Sinister (OS). The Intraocular Pressure (IOP) was 12 mmHg. The experienced retinal specialist concluded that this was a small melanoma close to the fovea and optic disc with some changes since 1981. Figures 1C-1E shows that due to the small size and the long observation time enucleation was considered, but not recommended (Figures 1A-1E).
Thereafter, the lesion was evaluated annually with fundus photos and ultrasound B-scan. The whole lesion became more pigmented. The SRF located inferior to the tumor disappeared in 1988. The visual acuity dropped to 0.04 and the area of the tumor increased, surpassing the superior arcade by one DD in 1991. Ultrasound of liver, liver function tests and chest X-ray were normal. In 1993, she was admitted due to acute nephropathy that presented with anuria with high creatinine and carbamide (urea) values, of 819 and 23 respectively (normal values female >18 years-old 45-109 and 3.1 mmol-7.9 mmol). Treatment with hypertonic infusion with sodium chloride and diuretic reversed the condition. The serum tests IgG and IgM were positive for Tularemia and the diagnosis epidemic nephropathy was established. The renal function normalized as assessed with creatinine (Figures 2A and 2B).
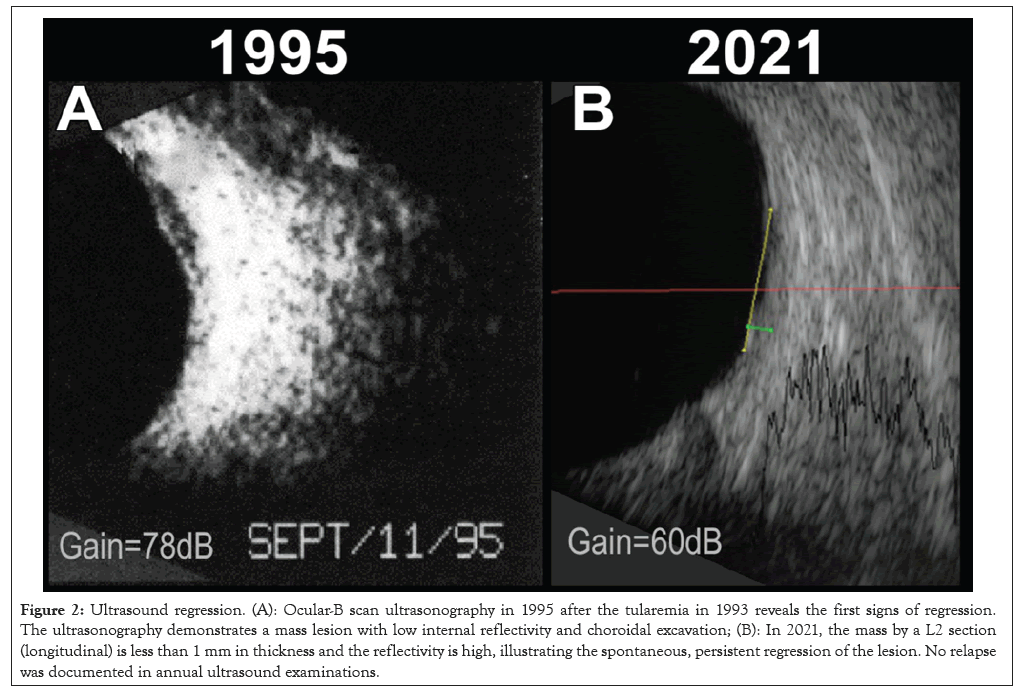
Figure 2: Ultrasound regression. (A): Ocular-B scan ultrasonography in 1995 after the tularemia in 1993 reveals the first signs of regression. The ultrasonography demonstrates a mass lesion with low internal reflectivity and choroidal excavation; (B): In 2021, the mass by a L2 section (longitudinal) is less than 1 mm in thickness and the reflectivity is high, illustrating the spontaneous, persistent regression of the lesion. No relapse was documented in annual ultrasound examinations.
In 1994, the tumor had less pigmentation in the central part and the lesion measured 1.4 millimeters (mm) in height. In 1995, the central vision in the left eye deteriorated to finger counting. Ultrasound B-scan demonstrated findings typically for a small UM with choroidal excavation and acoustic hollowness as shown in Figures 2A and 2B. The A-scan showed a typical high initial spike with abrupt drop to the ground line and a steep second spike. The central depigmented zone had increased slightly. No SRF or orange pigmentation was seen. Fluorescein angiography did not reveal any hot spots or subretinal neovascular vessels as shown in Figures 3A and 3B. Growth was documented in the area over years, minimal in height with the peripheral visual field preserved as shown in Figure 4. The lesion was probably a melanoma. Both enucleation and irradiation were considered, but not recommended due to the presumed negative effect on the optic nerve, the minimal changes during observation and most importantly, an enucleation had probably no influence of the future metastatic risk (Figures 3A and 3B and 4).
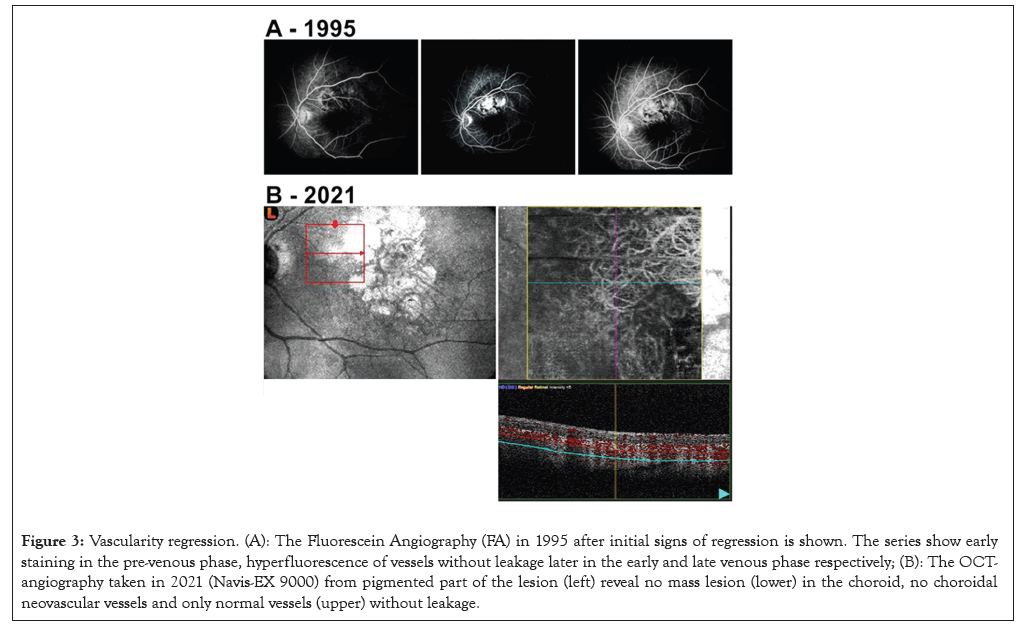
Figure 3: Vascularity regression. (A): The Fluorescein Angiography (FA) in 1995 after initial signs of regression is shown. The series show early staining in the pre-venous phase, hyperfluorescence of vessels without leakage later in the early and late venous phase respectively; (B): The OCTangiography taken in 2021 (Navis-EX 9000) from pigmented part of the lesion (left) reveal no mass lesion (lower) in the choroid, no choroidal neovascular vessels and only normal vessels (upper) without leakage.
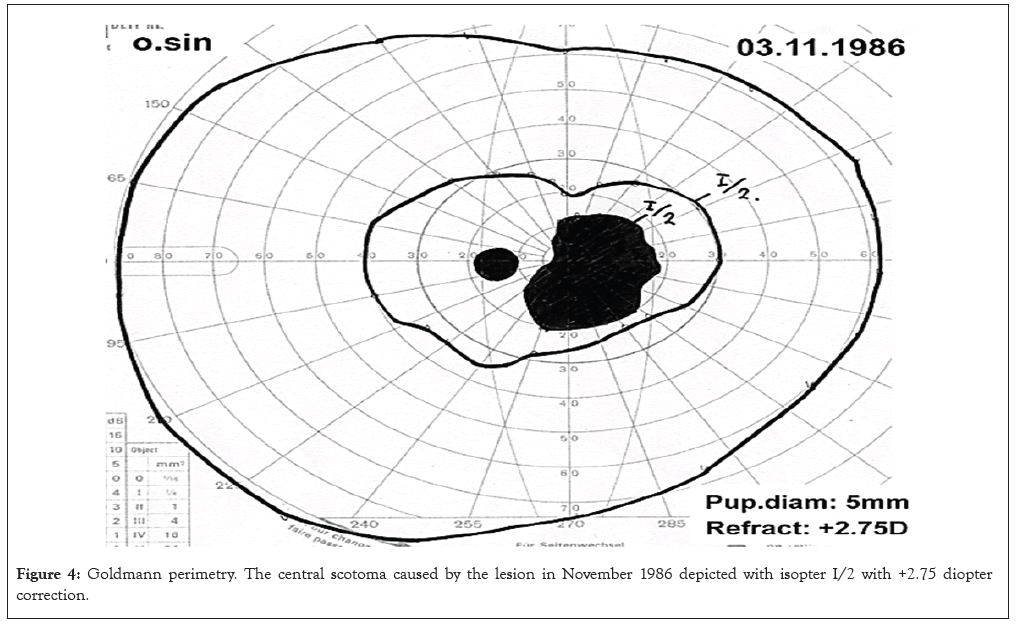
Figure 4: Goldmann perimetry. The central scotoma caused by the lesion in November 1986 depicted with isopter I/2 with +2.75 diopter correction.
The patient was monitored annually and with the last follow-up in 2023, the BCVA was 1.0 OD and finger counting OS. A flat chorioretinal scar persisted in the area of the previous pigmented mass, except for a small pigmented area in the upper central corner of the primary lesion measuring 2.0 mm × 2.7 mm on fundus image. The ultrasound B-scan revealed no mass.
The Optical Coherence Tomography (OCT) confirmed atrophy of choroidea with some vessels at the choroidea-sclera transition.
Due to sensory polyneuropathy, an orbital/cerebellar Magnetic Resonance Imaging (MRI) and a Computed Tomography (CT) abdomen/thorax were performed in 2022 with no pathological findings.
The mechanism behind regression of a UM in the absence of treatment is unknown, but a reasonable prediction could be cell-mediated immunity [10,11]. Necrosis, vascular insufficiency, inhibition of angiogenesis and hormone influence are other theoretical influencers. In cases of cutaneous melanomas, regression is associated with depigmentation clinically and degenerative tumor cells with inflammatory cells, melanin containing macrophages and dermal fibrosis histologically. The heterogeneity of the UM metastatic cells and/or the variation in microenvironment may be an explanation for the unappreciable response of ICB.
In our case, no halo nevi depigmentation sign has been observed circumscribing the tumor. Nevertheless, a halo was indicated on the temporal border in 1998. This sign supposedly indicates a more favorable prognosis. In 1993, our patient was treated for a severe infection. After 1995, no signs of progression of the eye lesion had been observed. Instead, a slow regression of the tumor mass and pigmentation has been seen starting from the central area. In three other cases, the regression was associated with ocular pain and inflammation [12].
The lesion increased in area for some years and then the SRF disappeared. The regression started with loss of pigmentation centrally, decline in visual acuity and gradually reduction of the mass of the lesion. Although it is impossible to be certain that our case observed over 41 years represent a UM without a histological or cytological confirmation of the diagnosis, we suppose that despite of a gradual regression our data are most consistent with a UM. Two medical centers with expertise in melanoma independently agreed in the slow growth phase based on clinical and imaging features, but elected to observe instead of enucleating. A pathologic confirmation of malignancy was not expected to influence the care of the patient or the outcome [13]. The case of Lambert, et al. [3] in 1986 with tissue confirmed pathology. A similar clinical course with no recurrence has been reported with 3-year follow-up [14]. We suppose that the severe Pasteurellae tularensis infection causing anuria had great impact on the evolution of the presumed melanoma disease.
The small lesion in the posterior pole was symptomatic, amelanotic with sub-retinal fluid and low internal reflectivity on ultrasound B-scan. These findings and substantial but slow growth before the tularemia infection in 1993 and increasing pigmentation over ten years were the basis for the melanoma diagnosis at two medical centers with expertise in posterior intraocular malignancies. Although a histological/cytological confirmation of the diagnosis is lacking, we still regard the melanoma diagnosis to be more consistent than a nevus due to the clinical findings. The severe inflammatory disease is presumed to be essential for initiating a nearly complete regression of clinically diagnosed melanoma with no recurrence documented over two decades. The absence of another malignant disease in the very long observation period supports our assumption that this unique case represents a spontaneous regression with no relapse.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Eide NA, Syrdalen P, Navaratnam J (2023) A 47-Year-Old Woman with a History of Tularemia and Regression of an Untreated Juxtapapillary Amelanotic Choroidal Malignant Melanoma More than Four Decades Following Initial Diagnosis. J Clin Exp Ophthalmol. 14:963.
Received: 30-Oct-2023, Manuscript No. JCEO-23-27183; Editor assigned: 01-Nov-2023, Pre QC No. JCEO-23-27183 (PQ); Reviewed: 15-Nov-2023, QC No. JCEO-23-27183; Revised: 22-Nov-2023, Manuscript No. JCEO-23-27183 (R); Published: 01-Dec-2023 , DOI: 10.35248/2155-9570.23.14.963
Copyright: © 2023 Eide NA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.