Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2021)
Aim of the study: To determine the impact of 3D Virtual Reality on the adherence of patients to three months of treatment for glaucoma.
Materials and methods: Randomized, single-blind clinical trial. Seventy patients were randomized to receive information about glaucoma via 3D Virtual Reality (3D group, 35 patients) or via printed material (control group, 35 patients). The mean measurement of both eyes from all patients was used to evaluate outcomes. The randomization was stratified to balance the number of patients using monotherapy or polytherapy between the groups.
At the first appointment, patients in the 3D Virtual Reality group watched a 3D video about glaucoma; patients in the control group received information via printed material. The primary outcome measures were Intraocular Pressure (IOP), Corneal Pachymetry, and Visual Field, performed at first and the three-month appointments.
Results: Neither the Corneal Pachymetry nor Visual Field changed after the three months of treatment; however, the overall IOP decreased (p=0.0001). IOP variation did not differ between monotherapy and polytherapy patients (p=0.15). Women had a trend toward better control of IOP than men, but the effect did not reach statistical significance (p=0.055). Even though overall IOP variation did not differ between the 3D and Control groups (p=0.25), the IOP decrease was higher in the 3D group than the control group, in monotherapy strata (p=0.006).
Conclusion: Our data showed that 3D virtual stimulation did not improve the three-month treatment adherence of glaucoma. However, it may improve adherence in patients at early stages or less affected by the disease, such as those in monotherapy. For those patients, we recommend further studies with larger sample sizes.
Glaucoma; Corneal Pachymetry; Intraocular Pressure
Glaucoma is the leading cause of irreversible blindness worldwide, and the number of affected individuals is expected to increase, mainly in Asia and Africa [1]. The disease has complex pathophysiology and goes unnoticed until severe visual field loss occurs [2]. Unfortunately, the treatment adherence in glaucoma is not ideal [3]. Moreover, the belief of the risk for vision loss impacts adherence to treatment [4].
3D virtual reality is a multisensorial experience that may be useful in medical learning, such as neurointervention [5], minimally invasive surgery [6], and ophthalmoscopy [7], as well as a therapeutic tool after stroke [8], and diabetes [9]. As a new technology, there has been no report on the effect of 3D virtual reality on adherence in the treatment of ophthalmological diseases until now.
The purpose of our study was to determine whether 3D virtual reality could improve adherence in the treatment of patients with glaucoma.
Ethics
The study was conducted following the Declaration of Helsinki. Our group was granted approval by the Ethics Committee on June 30th, 2017, (Brazilian Ethical Research Committee, number 2.149.268). If the specialists suspected glaucoma, they invited all of the eligible subjects to participate in the study. The patients then received all of the information and signed a consent form.
Inclusion and exclusion criteria
We included literate glaucoma patients with age equal to or above 18 in treatment on drops for glaucoma for at least six months. The minimal visual acuity was 20/40, according to the Snellen table. The exclusion criteria included an age of fewer than 18-years-old, illiterate patients, the demand for surgery during the three-month evaluation period, corneal diseases that interfered with the accurate measurement of intraocular pressure (IOP), and retinal diseases that impeded the quality of an optical tomography coherence exam (OCT) and computer perimetry. Newly diagnosed patients (less than six months) were also excluded from the study to avoid bias related to the disease’s initial understanding and treatment.
We allocated the patients into two groups. In the first group (3D), the patients watched an eight-minute 3D video about glaucoma and its consequences. In the second group (CONTROL), the patients received similar information via printed material. Our group provided both the video and leaflet information soon after the first appointment. We included the leaflet (Portuguese version, only) in the appendix section. The next section summarizes the leaflet’s main content; therefore, the readers can have a general idea of the amount and type of information offered to the patients.
According to the number of drugs employed in their treatment, the patients were also classified as either monotherapy (only one drug) or polytherapy (two or more different drugs used for treatment).
3D Video and leaflet
The patients in the 3D group watched an eight-minute, 3D video (Alcon Pharmaceutical, Novartis). The device (3D Glaucoma Simulator®) was attached to an Apple iPhone 5C (Apple, California). During the eight-minute-video, the audio describes general information about the disease, while the video gradually shrinks to simulate the progressive sight loss of glaucoma. The final seconds of the movie simulate the absence of peripheral vision. The original video is not available on the internet; however, one can easily find similar content under “glaucoma VR video.” A Brazilian company (MW2 Group®) developed the video’s Portuguese version, compatible with the app Google Cardboard®.
The leaflet is a twelve-paragraph, jargon-free-language text, where the patients get information concerning glaucoma definition, incidence, types, treatment importance, and consequences in the long-term for the eyesight. It is a supposed three to five minutesreading, with two figures one of one eye, explaining the effect of high intraocular pressure, and the second showing the narrowing of the visual field across the time.
A preliminary test was made in ten aleatory patients, five in 3D Video and five in leaflet information, to get feedback and adjust comprehension before data collection. Those patients were not included in the study dataset.
Randomization
Figure 1 shows the CONSORT flow diagram of the study. The site randomization.com provided tables for randomization. We decided to use blocks of two, four and six patients, to distribute the patients randomly. Sealed envelopes contained the designation of the patient’s group. We combined the strata of the randomization categorizing the patients concerning monotherapy and polytherapy to secure a similar number of candidates in each group. Three different specialists evaluated the patients at the first appointment. The specialists sent the eligible patients to a technician who randomized the patient either into the 3D or control group. In the technician’s room, the patient received the instructions for the relevant group. Therefore, all three ophthalmologists were blinded to the treatment. Three months later, the patients returned for reevaluation, and the technician repeated all the steps.
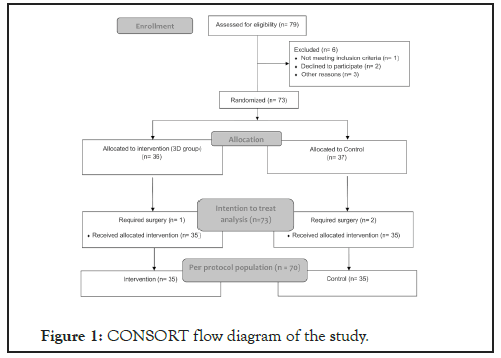
Figure 1: CONSORT flow diagram of the study.
Measurements
The IOP was the primary endpoint. The secondary endpoints included the visual field, pachymetry, and data from OCT. For intraocular pressure, the authors relied on the Goldmann tonometer, (Haag Streit). The technician measured all of the pressures in the time window between 9:00 AM and 2:00 PM. In the first appointment, three measurements were performed on each eye, and the mean value for each eye was calculated. The mean of both eyes was used as the value for each patient; in two patients only one eye was considered since they could not provide the measure of the other eye. The patients underwent the same process at the three-month appointment. A Topcon DRI – Triton was used to obtain the pachymetry measurements. The equipment offered the measure of the mean central corneal thickness. The visual field of the patients was measured by an Achromatic Perimetry Visual Field Humphrey 2-740 central 24-2 White White.
Sample size, standard deviations, and effect size
We aimed to detect the IOP difference between the groups at the three-month appointment with sensitivity of at least 1.5 mmHg. In a set of data obtained from patients in our four clinics, we estimated a standard deviation of 2.5 mmHg IOP in our glaucoma patients based on a survey performing during the first trimester of 2017. A difference of 1.5 mmHg between the groups, with the reported standard deviation, would represent a Cohen’s d of 0.625. As we focused on the impact of 3D stimulation, the effect size calculation relied on a one-tailed t-test. Therefore, to test the hypothesis that the groups were not equivalent, based on alpha less than 0.05 and a beta of less than 0.8, we required two groups of 33 patients each. The protocol included all randomized patients, according to the intention-to-treat argument.
Adherence, drop-outs, and missing data
The staff encouraged the strict treatment adherence of all of the patients in the protocol and communicated the potential harm of glaucoma. After being randomized, the patients followed the regular flow of appointments, experiencing the intervention (either 3D Virtual reality or leaflet) in the first assignment. According to the study design, six more patients (ten percent) were included to cover attrition. Software-driven multiple imputations dealt with missing data, and the linear regression was used for continuous variables.
Statistical analysis
For statistical analysis, our group used Stata software (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP). We tested all of the variables for normality distribution, using the Shapiro-Wilk test. For intergroup analysis, we used an independent t-test for the independent means. Conversely, to compare intragroup changes, we used a dependent t-test. T-tests required the previous proof of normal distribution of the variables. We aimed to provide confidence intervals and the effect size whenever possible. The analysis of covariance (ANCOVA) was performed to identify possible factors involved in the variation of endpoints and from the first appointment to the three-month visit. We considered the equality of the control and 3D groups as the null hypothesis concerning the endpoints. To reject the null hypothesis, we set a beta of 0.8 and an alpha error of less than 0.05 as the cut-offs.
From July to October 2018, seventy-three patients were enrolled in the study. Three patients required surgery and were removed from the protocol. Therefore, we included a total of 70 patients, with thirty-five patients in the 3D group and thirty-five in the Control group. Table 1 shows the demographics of the patients.
| 3D | Control | Total | p-value | |
|---|---|---|---|---|
| Number | 35 | 35 | 70 | 0.38 |
| Age (mean) | 67.9 | 65.6 | 66.8 | |
| Age ≤ 67 (n/%) | 18/25.71 | 19/27.14 | 37/52.86 | 0.81 |
| Age>67 (n/%) | 17/24.29 | 16/22.86 | 33/47.14 | |
| Female (n/%) | 20/28.57 | 16/2.86 | 36/51.43 | 0.34 |
| Male (n/%) | 15/21.43 | 19/27.15 | 34/48.57 | |
| Monotherapy (n/%) | 11/15.71 | 12/17.14 | 23/32.86 | 0.80 |
| Polytherapy (n/%) | 24/34.29 | 23/32.86 | 47/67.14 |
Table 1: Patient characteristics in both treatment groups (3D and control). Chi-squared test provided the p-value.
Over three months, the overall IOP dropped from 14.65 ±
3.03 mmHg, to 13.45 ± 2.32 mmHg, p=0,0001. The mean IOP
variation at the three-month exam was 1.21 ± 2.29 mmHg, 95%
CI (0.67-1.75). The mean pachymetry and the mean visual field
did not change during the three-month-study. Table 2 shows the
variables at first and three-month-appointment.
| Initial appointment (mean, SD) | 3-Month appointment (mean, SD) | 3-Month variation (mean, SD) | p-value | ||
|---|---|---|---|---|---|
| Mean Pachymetry (µm) | 536.72 ± 34.95 | 537.3 ± 25.45 | 0.56 ± 24.6 | 0.43 | |
| Mean Visual Field (PSD, dB) | 4.09 ± 3.14 | 3.68 ± 2,51 | 0.41 ± 3.01 | 0.87 | |
| Mean Intraocular Pressure (mmHg) | 14.65 ± 3.05 | 13.51 ± 2.28 | 1.14 ± 2.23 | 0.0001 | |
Table 2: Three-months change of the main variables.
3D virtual reality did not affect the overall mean IOP reduction at the three-month appointment. Analysis of covariance (ANCOVA) was performed, using the mean IOP at three-monthappointment as the response variable, baseline IOP means as the covariate and group and age as factors. ANCOVA did show statistical significance for age (Prob>F=0.029) but did not show statistical significance for group (Prob>F=0.73).
We compared the mean three-month IOP variation between both the 3D and control groups and the results supported the ANCOVA findings (p=0.36). The comparison of the mean three-month IOP variation according to the number of drugs showed no difference between monotherapy patients compared to polytherapy patients (p=0.15). When the comparison involved gender, there was a trend toward a higher IOP reduction among women, but this effect did not reach statistical significance (p=0.055). We stratified the patients into two groups by age: patients equal to or less than 66 years and patients above 66 years. There was no difference between age (p=0.09). Table 3 shows the IOP variation in the subgroups.
| Mean Initial IOP (mmHg) mean ± SD | Mean 3-M IOP (mmHg) mean ± SD | Difference (mmHg) mean ± SD | Effect size based on the mean comparison of the difference (Hedge's g) | p-value | ||
|---|---|---|---|---|---|---|
| Age ≤ 66 | 14.82 ± 3.29 | 13.04 ± 2.59 | 1.5 ± 2.17 | 0.32 | 0.09 | |
| Age>66 | 14.53 ± 2.91 | 13.82 ± 2.02 | 0.88 ± 1.64 | |||
| Men | 14.65 ± 2.96 | 13.66 ± 2.38 | 0.76 ± 1.72 | 0.38 | 0.054 | |
| Women | 14.65 ± 3.17 | 13.37 ± 2.21 | 1.48 ± 1.99 | |||
| Monotherapy | 15.24 ± 2.73 | 13.81 ± 1.74 | 1.47 ± 2.16 | 0.27 | 0.15 | |
| Polytherapy | 14.35 ± 3.16 | 13.36 ± 2.49 | 0.96 ± 1.73 | |||
| 3D | 14.85 ± 2.60 | 13.58 ± 1.50 | 1.28 ± 1.99 | 0.16 | 0.25 | |
| Control | 14.45 ± 3.45 | 13.46 ± 2.87 | 0.98 ± 1.79 | |||
Table 3: Three-months intraocular pressure variation in the study subgroups. The comparison between each subset (age ≤ 66 versus age >66, men versus women, monotherapy versus polytherapy and 3D versus control) was used to determine an effect size and a p-value. IOP-Intraocular pressure; 3M IOPIntraocular pressure at the three-month appointment.
Even though the overall three-month variation of IOP in the 3D and control groups did not differ, we noticed a higher IOP reduction of the 3D group in monotherapy patients. The IOP dropped 2.40 ± 1.66 mmHg, 95% CI (1.29-3.52) in the 3D group (n=11), versus 0.62 ± 2.73 mmHg, 95% CI (-0.83-2.06) in the control group (n=12), in this subset of patients (p=0.02), (Figure 2). The IOP reduction in the 3D and control group did not differ in the subset of patients on polytherapy (p=0.78). Appendix Figure 1 shows Portuguese-version leaflet showed to the patients in the control group and Table 4 shows the p-value of 3 months variation and subgroup comparison.
| Initial appointment (mean, SD) | 3-Month appointment (mean, SD) | 3-Month variation (mean, SD) | p-value (3-month variation) | p-value (subgroup comparison) | ||
|---|---|---|---|---|---|---|
| overall IOP (mmHg) | 14.65 ± 3.05 | 13.51 ± 2.28 | 1.14 ± 2.23 | 0.0001 | ||
| male | 14.65 ± 2.96 | 13.66 ± 2.38 | 0.99 ± 2.28 | 0.02 | 0.054 | |
| female | 14.64 ± 3.17 | 13.37 ± 2.21 | 1.28 ± 2.20 | 0.0014 | ||
| ≤ 67 yo | 14.85 ± 3.38 | 13.30 ± 2.43 | 1.55 ± 2.56 | 0.0008 | 0.66 | |
| >67 yo | 14.42 ± 2.68 | 13.74 ± 2.11 | 0.68 ± 1.71 | 0.03 | ||
| monotheraphy | 15.25 ± 2.79 | 13.81 ± 1.78 | 1.44 ± 2.44 | 0.0096 | 0.14 | |
| polytherapy | 14.35 ± 3.16 | 13.36 | 0.99 ± 2.13 | 0.0026 | ||
| control | 14.85 ± 2.63 | 13.56 ± 1.52 | 1.28 ± 2.24 | 0.018 | 0.51 | |
| 3D | 14.45 ± 3.45 | 13.46 ± 2.87 | 0.99 ± 2.24 | 0.0129 | ||
Table 4: Three-months variation and subgroup comparison.
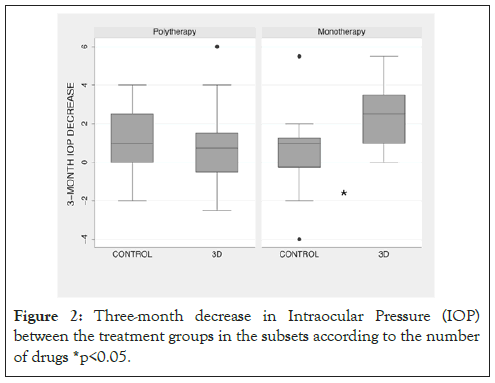
Figure 2: Three-month decrease in Intraocular Pressure (IOP) between the treatment groups in the subsets according to the number of drugs *p<0.05.
Our data suggest that 3D virtual reality did not affect adherence in the three-month treatment of glaucoma. However, it may improve adherence in the early stage or well-controlled disease, such as in the monotherapy subset of patients. The mean reduction of IOP of 2.4 mmHg, in the 3D group, compared to 0.62 mmHg in the control group, was statistically significant and clinically relevant, despite the small size of this subset (n=23). The present study is the first to describe 3D virtual reality in treatment adherence for an eye condition, particularly for glaucoma. However, our data did not demonstrate the influence of such an intervention in glaucoma patients using polytherapy.
The treatment of chronic, oligosymptomatic disease is challenging for two main reasons: the side effects of some drugs and the cost for the patients. The lack of symptoms and limitations, at least in the early stages of the disease, is another factor that contributes to poor treatment adherence. In a cohort of 16907 patients who were treated for different conditions, Blaschke showed that 40% of the patients abandoned the treatment after one year, and 4% did not even start the treatment [10]. The lack of symptoms in the initial stages of the disease is also a cause of poor treatment adherence among glaucoma patients, combined with the cost of medication, age, and difficulty of the daily administration of the eyedrops [11].
3D virtual reality may interfere with “health-related behaviors through motivational reinforcement,” according to Ershow [9]. His paper highlighted the role of 3D virtual reality in the education and motivation of patients with diabetes. Our 3D video was more alarming than motivational since the patients gained a more realistic impression of how much glaucoma could impair their sights. We hypothesized that the 3D video could improve adherence because the patients would understand the potential sight impairment of glaucoma in a very realistic way; which is challenging to communicate through leaflets and personal explanations. Furthermore, the patients in Monotherapy, assumed to have less affected eyesight, are those who get the best benefits from 3D technology, since its effects are directly related to the sense of vision.
Impressively, the data that we show in the present study represent the role of 3D video during the initial phase of the treatment – the first three months. Authors have shown that adherence decreases over time, with an abrupt decrease in the first six months [12]. We should probably repeat the use of the 3D video in a second appointment and analyze the impact in six months, or an even a more significant amount of time, in a future study.
Our findings did not show an improvement in adherence to polytherapy patients. Adherence is poorer in polytherapy patients (3, 13). In Kim’s study, 30% of the patients were non-adherents, especially in males and in patients with higher numbers of daily required medications; Joseph and Pasquale presented the “simplified drug regimen” as a strategy to improve adherence. We believe in the involvement of economic factors, as well as drug side effects impair adherence in polytherapy patients. Moreover, older individuals are not as proficient with advanced technology as younger patients are.
Our study showed a trend for better control among women. The staff believes that females take prescription adherence more seriously than males, at least in our country. Our data support Kim’s findings [3]; however, the results go against the reports of a paper referenced above from Nordstrom and colleagues [12], who found a higher risk of treatment discontinuation among females (in diagnosed but not suspected glaucoma), in four regions of the United States. We believe that sociocultural and economic differences mays explain such differences [13].
We aimed to design a robust study from the beginning that strictly complied with the CONSORT statements [14], including the design, ethics, sample size, type of randomization, maintenance of blinding, missing data replacement, and statistical analysis. The mean IOP decrease was higher in 3D patients compared to control patients in monotherapy patients only; however, we must note that this subgroup monotherapy was smaller than the polytherapy subgroup (23 versus 47 patients). The small sample size of monotherapy patients can compromise the extrapolation of the finding to the entire population. Moreover, the study lacks a way of measuring compliance, as a drop dispenser device, for example. Unfortunately, we only rely on our population’s homogeneity regarding education, sex, and race to extrapolate our data. However, the difference between the groups is big enough to justify future research on the subject, with a larger sample of patients, more extended periods of study, and a way of measuring adherence. We also believe in the positive cumulative effect of multiple 3D virtual simulations on treatment adherence in Glaucoma patients in the long term. Many authors have highlighted different processes to educate patients and therefore improve glaucoma treatment adherence. Shah and Shaikh described a method to improve the understanding of glaucoma by trained volunteers [15]. They also commented on other methods such as phone calls, printed materials, and non-3D videos. We believe the 3D virtual reality approach provides a rich medium that is superior to the previous techniques regarding education.
The present study aimed to detect the three-month IOP variation. Therefore, we were unable to determine the response of the groups at six months or after more extended periods and whether the IOP decrease could be maintained after three months. Finally, we speculate that there would be an additive effect of the 3D virtual reality if it were used in subsequent appointments, as we pointed previously.
The failure to control glaucoma is frustrating. Along with the importance of selecting the best treatment, poor adherence may result from an incomplete understanding of the disease and its consequences. Moreover, poor control of glaucoma decreases the quality of life of the patients over time [16].
When we designed the present study, we aimed to compare two methods of communicating information about the disease; both methods were presented a clear and contained similar content. The study may raise questions about how we can directly relate the outcome (three-month intraocular pressure decrease) and treatment adherence. According to Schwartz and Quigley [4], it is almost impossible to determine adherence accurately in Glaucoma patients. Therefore, we assumed that any benefit in the primary endpoint would be assigned to better treatment adherence since the patients came from a stratified, randomized sample.
Our data show that 3D virtual reality did not change treatment adherence in Glaucoma patients; however, it may be useful in particular subgroups of patients, such as those in the early stages or less affected by the disease. Because of the importance of glaucoma to public health, new studies are required to bring this technology into clinical practice. 3D virtual reality is a new promising tool that may help people overcome diseases like glaucoma and other conditions with poor treatment adherence.
Citation: Raiza ACP, Balbino M, Takiuti JT, Takahashi VKL, Santo AD, Minelli E, et al. (2021) 3D Virtual Reality may Enhance Adherence in Glaucoma Patients Using Monotherapy: A Randomized Clinical Trial. J Clin Exp Ophthalmol. S12:001.
Received: 06-Jan-2021 Accepted: 21-Jan-2021 Published: 28-Jan-2021 , DOI: 10.35248/2155-9570.21.s12.001
Copyright: © 2021 Raiza ACP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.