Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Case Report - (2020)Volume 11, Issue 4
Xeroderma Pigmentosum (XP) a unique disorder, inherited as an autosomal recessive genodermatosis. Characterized by photosensitivity, freckly pigmented changes, premature skin ageing, telegiectasis, warty and papillomatous growth and malignant tumor development in later stage. Results from mutation in seven nucleotide excision repair gene (XPA to XP-G complement groups) and post replication repair defect (XP-Variant). This report aims to acknowledge the unique combination of XP along with neurological defects in a patient of acute myeloid leukemia.
Xeroderma pigmentosum; Acute myeloid leukemia; Meningiomas
Xeroderma Pigmentosum (XP) an autosomal recessive genetic disorder of DNA repair in which the ability to repair damage caused by UV light is deficient. The basic genetic defect underlying the clinical manifestations is in which nucleotide excision repair (NER) enzyme are mutated leading to a reduction in or its elimination. NER, major DNA repair pathway in eukaryotic cells.The human genome is constantly attacked by endogenous reactive metabolites, therapeutic drugs and a plethora of environmental mutagens that impact its integrity. Thus it is obvious that the stability of the genome must be under continuous surveillance. NER is responsible for the repair of bulky DNA adducts such as UV-light-induced photo-lesions, intra-strand cross-links and large chemical adducts generated from exposure to genotoxic agents. An impaired ability to repair damaged DNA leads to genomic instability and its consequences in the form of different clinical entities like XP, Cockyne Syndrome and Trichothiodystrophy.
A 33 years old female patient brought by her parents with the complaints of high grade fever, hyper and hypopigmented areas all over the body, severe pain in the eyes on exposure to light, inability to open the eyes and failure to thrive.
On further questioning, they stated when she was 3 years old, developed multiple fluid filled blisters over sun exposed areas which were associated with itching, burning and photosensitivity. When the blisters ruptured, healed with scarring and depigmentation. She suffered multiple episodes of the same throughout her life. History of watering, burning and swelling over eyes and photophobia.
On examination, patient was short stature, her head circumference was 3rd percentile of her age, slurred speech. Cutaneous examination showed multiple freckling, depigmented and hyperpigmented areas on the face and other exposed parts on her body along with scarring of the skin (Figure 1). Ocular examination: intense photosensitivity, corneal opacities, lid basal cell carcinoma (Figure 2). Neurological examination: hyporeflexia, absent deep tendon reflex, inability to walk.
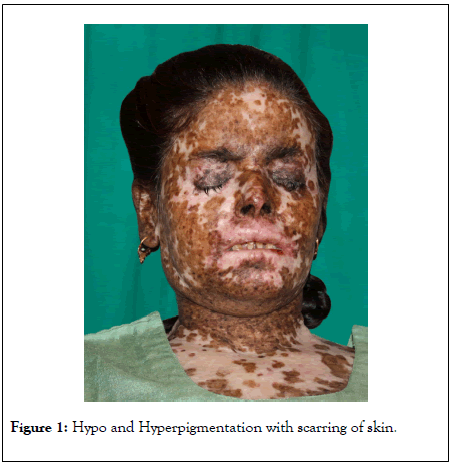
Figure 1: Hypo and Hyperpigmentation with scarring of skin.
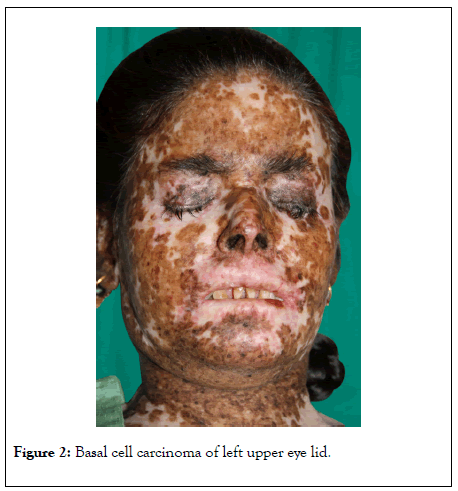
Figure 2: Basal cell carcinoma of left upper eye lid.
A complete blood count demonstrated severe anemia (hb-3.1 gm/dl), TLC-2700, thrombocytopenia 15,000.
Urine: negative for porphyrins. Wood’s lamp examination was negative for porphyria (Figure 3).
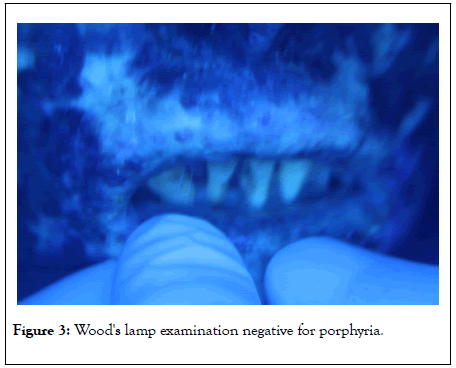
Figure 3: Wood's lamp examination negative for porphyria.
Radiological evaluation showed X-ray skull showed bilateral thickening of inner and outer table of skull and upon CT head we found bilateral meningiomas (Figure 4).
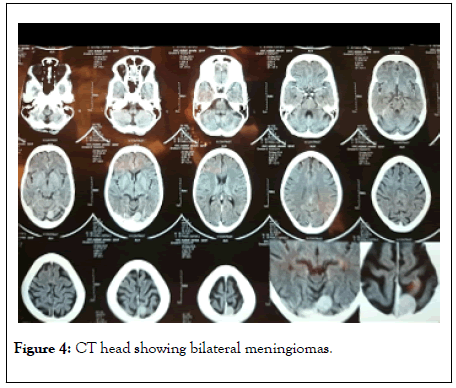
Figure 4: CT head showing bilateral meningiomas.
Ultrasonography of abdomen showed atrophic uterus with tiny bilateral ovaries.
Bone marrow examination: changes consistent with Acute Myeloid Leukemia, more than 20% blast cells of myeloid origin were seen.
On these grounds diagnosis of neurological variant of XP with acute myeloid leukemia (AML) and bilateral meningioma was made.
Molecular events associated with nucleotide excision rehabilitation of UV-induced DNA damage
UV irradiation composed of both ultraviolet A spectrum (320-400 nm) and the UVB spectrum (280-320 nm), UVB plays a more pivotal role in the etiology of XP. UV irradiation causes two major photoproducts in DNA: cyclobutane pyrimidine dimers (CPD) and (6-4) pyrimidine-pyrimidone photoproducts (6-4PP). These DNA lesions influence cellular death, aging, mutagenesis, and carcinogenesis when they are not fully rectified by the DNA repair machinery of the cells. XP arises from mutations of XP genes (XP-A to XP-G) resulting in impaired NER.
Defects in the eighth XP gene do not affect NER, Instead these so-called XP variants (XP-V) have problems replicating DNA containing ultraviolet-induced damage. XP-V patients have normal NER, but mutations in DNA polymerase η (POLH) cause a defect in the ability to replicate DNA containing unrepaired damage [1]. The NER pathway is the mechanism responsible for rehabilitating UV-induced helix- distorting lesions of DNA. NER comprises two sub pathways: global genome NER recognizes and rehabilitates UV-induced DNA lesions in non-transcribed DNA throughout the genome, while transcription-coupled NER is initiated by damaged DNAinduced apprehend of transcribing RNA-polymerase II on the transcribed strand of an active gene [2-4].
Consanguinity
Implicated as an etiological factor. This has been reported to varying degrees of up to 92.8% in XP patients in Libya [5]. Other studies reported from Egypt, Pakistan, and Nigeria have a high incidence of XP [6]. However, a case report by Stephanic Christen et al. showed a family where the uncle and nephew were affected by XP [7].
Skin
Sunburn is a normal response to UVR exposure. The confluent and well-demarcated, erythematous, edematous, and tender reaction is caused by vasodilation and inflammation. Patients with XP suffer incremented susceptibility to severe and prolonged sunburn that mayrelate to the persistence of UVR photoproducts in the DNA. Only 50% of XP patients suffer from griveous and perpetuated sunburn reactions, remaining 50% present with lentigines at sun-exposed sites, and have sunburn reactions that are normal for their skin type [9-11].
Skin cancer
Incidence in dark-skinned people customarily are lower compared to light skinned people most probably owing to the photoprotective properties of melanin. But dark-skinned and light-skinned people with XP, have kindred incidences of skin cancer accentuating the essential role of DNA repair mechanisms even in the presence of defence by melanin [12]. Areas of hyper- and hypopigmentation results in the absence of rigorous protection from the sun, followed by accelerated photoageing, warty lesions, in situ melanocyte, keratinocyte malignancy, and eventually multiple basal cell carcinomas and invasive squamous cell carcinomas and melanomas [13]. XP patients have a >10,000-fold incremented risk of developing non-melanoma and a 2,000-fold incremented risk of melanoma skin cancer inpatients under 20 years of age.
Ocular
UV induced DNA alteration to epithelial cells of the conjunctiva, cornea, and the eyelid, causes ocular abnormalities in about 40%-80% of patients with XP.
Damage to eyelids and periocular skin result in the development of cicatricial skin changes as well as skin cancers, which may require excision.Ocular surface can develop UVR-related damage including dry eye, conjunctival injection, and inflammation as well as development of premature pingueculae and pterygia. Ocular surface cancers, mainly squamous cell carcinomas, have additionally been reported in patients with consequential UVR exposure and poor ocular photoprotection [14].
Neurodegeneration
Typically follows skin symptoms and do not arise before 2 years of age. Postulated as a consequence of defective DNA associated with mutated XP genes, neurons accumulate endogenous genotoxic-induced DNA damage and undergo apoptosis leading to loss of neurons.
Neurological abnormalities are only visually perceived in individuals with defects in complementation groups XPA, XPB, XPD, and XPG. The most astringent neurological deficits are visually perceived in DeSanctis-Cacchione syndrome while the most frequent neurological abnormality is a loss of highfrequency hearing. The incidence for central nervous system tumours (CNS) is additionally ten times higher than in the normal population. Astrocytomas, medulloblastomas, glioblastomas, and malignant schwannoma are among the CNS tumors.
Oral
SCC most frequently affects the posterolateral and ventral surfaces of the tongue and floor of the mouth of elderly which runs an aggressive course. By contrast, XP associated SCC affects the tip of the tongue younger than 20 years of age and runs a gradually progressive course. The precancerous and cancerous lesions of the tip of the tongue, are presumed to be induced by UV radiation. Leukoplakia, erythroplakia, and SCC of the tip of the tongue, actinic cheilitis, and SCC of the lips are associated with XP [15].
In 1870, Moritz Kaposi first utilized the term “xeroderma” to characterize the dry, dyspigmented skin that is the first indefinite cutaneous change observed in patients with this disease.
Autosomal recessive disease with sun sensitivity, photophobia, early onset of lentigens and freckling with increased incidence of cutaneous neoplasm. The first XP case with neurological signs was described by Dr. Albert Neisser and in 1932, DeSanctis and Cacchione helped coin the term “ DeSanctis-Cacchione syndrome ” to apply to cases of XP with severe neurological deficiency, characterized by growth failure, severe and progressive neurological deterioration, cachexia, retinal degeneration, and photosensitivity. The causes of the neurological abnormalities are yet not recognized. It has been suggested that it may be due to oxidative DNA damage engendered during normal metabolism in the central nervous system. This type of damage may be rehabilitated by NER.
Thus in the absence of normal functional repair by NER, neurological deficit takes place. Neurological manifestations typically follow skin symptoms in the natural history of the disease and do not arise before 2 years of age. EEG studies substantiate a spectrum of encephalopathy from mild-to-severe. Cerebellar signs manifest conventionally between age 4 and 16 years, customarily dysarthria and difficulties with balance. Ataxia and areflexia follow suit; EMG studies show evidence of axonal sensory and motor neuropathy although this is not customarily optically discerned before the second decade of life.
XP patients have a >10,000-fold incremented risk of developing non-melanoma and a 2,000- fold incremented risk of melanoma skin cancer in patients under 20 years of age.
There are three ways in which the eyes can be affected in XP; 1: UVR exposure resulting in DNA damage of the eyelids and periocular skin; 2: UVR exposure resulting in DNA damage of the ocular surface and 3: The ocular manifestations of neurodegeneration. Even patients with few ophthalmic signs commonly describe photophobia, which is the earliest presenting ophthalmic symptom of XP. These leads to damage of eyelids and periocular skin which further result in the development of skin cancers.Ocular surface cancers, mainly squamous cell carcinoma was recognized in patients with consequential UVR exposure and poor ocular photo protection.
Our patient had history of acute sunburn with blister formation at the age of 3 years, which correlates well with literature where photosensitivity mentioned at the age of two years.
Our patient had classical cutaneous and ocular features of XP with neurological manifestations in the form of hyporeflexia, absent deep tendon reflexes, slurred speech, difficulty in walking. This classifies our case as XP with neurological features. Unique findings in our case were small head circumference, atrophic uterus with tiny bilateral ovaries on USG abdomen and presence of bilateral meningiomas.
We have reviewed all published cases of malignancies related with XP reported in literature. Out of various associations of malignancies which were studied, cutaneous and ocular malignancy were most categorically described.
Normal individuals harboring XPD polymorphisms are at increased risk for developing acute lymphoblastic leukemia and acute myeloid leukemia (AML).
In a recent review, Janjetovic S et al. [14] reported a 33-year-old male patient with XP who was diagnosed with acute megakaryoblastic leukemia with a complex karyotype, as in our patient we found acute myeloid leukemia with xeroderma pigmentosum. XPD mutation in the patient may have contributed to the emergence of his high-risk AML.
Rohan Shetty et al. [15] also reported a case of a 13 year old girl with xeroderma pigmentosa who developed multiple cutaneous malignancies in the face and upper limb.
To our best knowledge XP coexisting with Acute Myeloid Leukemia and bilateral meningioma is not reported till date which is seen in our case. This paper describes an atypical case of Xeroderma Pigmentosum in a 33 year old unmarried female with no family history who had signs and symptoms since the age of 3 years. Her extensive and striking UV-induced skin and ocular damage are tragic evidence of the consequences of the disease, and of inappropriate medical care from an early age. Besides these symptoms patient did not present with any visible distinct features of meningioma like seizures, unintentional movements- twitchings or personality and memory changes which was diagnosed by CT, yet simultaneously the patient showed features of neurodegeneration like short stature, hyporeflexia and absent deep tendon reflex from the onset of puberty.
In our case, patient attempt for medical advice very late, during which damage had already occurred lead to failure of initiation of therapy and unpredicted loss.
Mutations in different DNA repair genes can be associated with the same clinical phenotype and conversely different mutations in DNA repair gene can be associated with different clinical phenotype. Our case maybe a result of multiple mutations in a single DNA repair gene and hence presented here because of its rarity.
There are very few cases of XP reported in literature from India. Reporting every case might help us to know the incidence and prevalence of XP in India which is yet unknown.
Citation: Mian NZ, Kushwaha P (2020) Xeroderma Pigmentosum with Acute Myeloid Leukemia and Meningiomas: Review of Literature. J Clin Exp Dermatol Res. 11:524. DOI: 10.35248/2155-9554.20.11.524
Received: 30-May-2020 Accepted: 15-Jun-2020 Published: 22-Jun-2020 , DOI: 10.35248/2155-9554.20.11.524
Copyright: © 2020 Mian NZ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.