Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Commentary - (2022)Volume 13, Issue 1
Cardiovascular disease is a significant long-term complication of uncontrolled hypertension, diabetes, and obesity, all of which co-occur in Metabolic Syndrome (MetS). The ongoing COVID-19 pandemic has highlighted the deleterious effects of MetS, as incidences of major cardiovascular complications such as thromboembolic events and myocardial infarction are significantly higher in patients with MetS. Several features of the myocardial tissue-level response to MetS, including a pro-inflammatory background, altered myocardial coagulation, and disturbances in the Renin- Angiotensin-Aldosterone system may explain the increased vulnerability of COVID-19 patients with MetS to deleterious cardiac effects.
Metabolic syndrome; COVID-19; Cardiovascular disease; Coagulation; Inflammation; Renin-angiotensinaldosterone system
Many severe complications of COVID-19 infection, including myocarditis, myocardial infarction, and arterial and venous thromboembolism are more common in patients with obesity, hypertension, and diabetes, all of which are components of Metabolic Syndrome (MetS) [1,2]. Hypertension, obesity, and diabetes are among the leading risk factors for serious illness that necessitates hospitalization [3]. Potential interactions between MetS and SARS-CoV-2 that may cause severe heart effects require further investigation. We emphasize three main factors of the myocardial tissue-level response to MetS that may help explain inferior cardiovascular outcomes following COVID-19 infection and suggest early pharmacological treatments for cardiovascular complications in the increasingly common MetS and COVID-19 comorbidity.
The pig animal model, which recapitulates the hallmark criteria of human MetS, offers a putative mechanism for the worse COVID-19 outcomes seen in patients with obesity, hypertension,and diabetes. MetS significantly decreases expression of myocardial Toll-Like receptors (TLRs), most noticeably TLR [3-8]. TLR-induced transcription of the activator of interferon alpha and beta production and anti-viral regulatory factor 3 (IRF3) is also significantly reduced in MetS [8]. At the same time, the pro-inflammatory arachidonic acid cycle enzymes phospholipase A2, phospholipase beta, Cyclooxygenase 1 (COX-1), Cyclooxygenase 2 (COX-2) and thromboxane synthase all exhibit significantly elevated expression [4]. COVID-19 infection induces COX-2 expression in human cell culture and mouse models [5], further adding to the already dysregulated inflammatory environment in the MetS myocardium. Currently COX inhibitors, specifically Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) like ibuprofen and meloxicam, have been tested as potential treatments for COVID-19 infection. While NSAIDs do not appear to reduce viral entry or replication, they may help to reduce the severity of the systemic inflammatory storm often observed in severe COVID-19 infections that is associated with end-organ damage [5]. In addition, given that SARS-CoV-2 is an enveloped single stranded RNA virus, low myocardial levels of TLR [7,8], and IRF, which are key components of innate antiviral immunity, may reduce myocardial capacity to initiate a strong response to COVID-19. Given the decreased baseline levels, pharmacologic targeting of TLRs may have potential therapeutic benefit in COVID-19 for the myocardium in patients with MetS.
The balance between coagulation and fibrinolysis in the myocardium tips in favor of excessive coagulation, with increased expression of clotting factors (VIII, IX, X and XIIIa) and promoters of platelet aggregation (ADP, platelet P2Y1 receptor, prostacyclin synthase) alongside decreased expression of coagulation inhibitors and fibrinolytics such as protein S, protein C, thrombomodulin, and tissue plasminogen activator tPA [4]. Patients with COVID-19 infection are at increased risk for micro and macrovascular thrombotic complications, the most frequent being pulmonary embolism with deep vein thrombosis [6]. Specific mechanisms of COVID coagulopathy remain unclear, although most hypotheses propose a link between hyperactivation of the immune system, complement system, and induction of the coagulation cascade [7]. The lectin and alternative complement pathways are particularly vulnerable to COVID-19 induced hyperstimulation, leading to excessive downstream activation of effector enzymes such as MASP-2 from the lectin pathway and disinhibition of C3 convertase production from the alternative pathway [8,9]. In addition to elevated factor XIIIa, pig models of MetS display increased MASP-2 and factor D levels in the myocardium in MetS, suggesting a baseline tendency towards increased lectin and alternative pathway activity [4]. Hence in the context of MetS, COVID-induced activation of complement pathways may exacerbate complement and coagulation dysfunction in the myocardium.
RAAS refers to the renin-angiotensin-aldosterone system. Angiotensinogen (AGT), Angiotensin Converting Enzyme (ACE), and the Angiotensin Type 1 Receptor (AGTR1) AGTR1 are all upregulated in MetS while ACE2 and Apelin (APLN) are down regulated [8]. Furthermore, the ratio of ACE/ACE2 is substantially elevated in MetS [5]. Binding of the SARS-CoV-2 spike protein to ACE2 leads to cellular entry, internalization of ACE2 and down regulation of ACE2 expression [10,11]. Angiotensin II binding AGTR1 represents the “classical arm” of RAAS, promoting vasoconstriction, fibrosis, oxidative stress, and insulin resistance. In the “protective” arm, ACE2 drives conversion of angiotensin II to angiotensin [1-7], which acts on AGTR2 and the MAS receptor to promote vasodilation and inhibit cell proliferation and inflammation [10]. Decreased ACE2 in COVID-19 infection impairs the RAAS protective arm and contributes to several symptoms of classical arm hyperactivity such as treatment-resistant hypertension, hyperglycemia, atherosclerosis, and inflammatory storm. Further evidence for this hypothesis comes from observations of increased angiotensin II in patients with COVID-19 infection and ACE2 knockout mice that exhibit significant left ventricular dilation and impaired cardiac contractility [12]. COVID-19 infection may worsen preexisting ACE2 deficiency in MetS through further ACE2 internalization and down regulation. Some evidence suggests that ACE inhibitors and ARBs reduce risk of severe disease in patients with COVID-19; however, no formal recommendations currently exist [13]. It is possible that treatment with ACE inhibitors or Angiotensin Receptor Blockers (ARBs) might be of particular benefit for COVID-19 patients with MetS.
The myocardial MetS environment and its correlation to COVID-19 is graphically depicted in Figure 1.
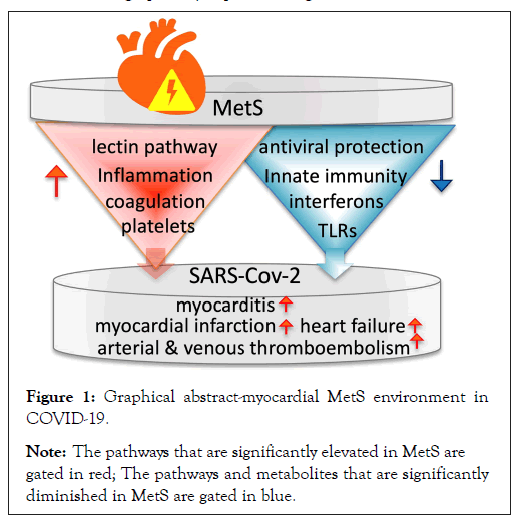
Figure 1: Graphical abstract-myocardial MetS environment in
COVID-19.
Note: The pathways that are significantly elevated in MetS are gated in red; The pathways and metabolites that are significantly diminished in MetS are gated in blue.
Patients with MetS are particularly vulnerable to cardiovascular complications of COVID-19 infection. This is likely due to a variety of factors, including a baseline pro-inflammatory myocardial state, heightened propensity for developing coagulopathy, and excessive activation of the classical arm of RAAS at baseline. COVID-19 infection exacerbates all these features through mechanisms that sometimes appear to mimic MetS-related pathophysiology. It is possible that targeted interventions aimed at mitigating deleterious effects of each of these the three key features of MetS discussed here may improve outcomes of COVID-19 infection in patients with MetS.
We would like to acknowledge the support of the following two grants: R91HL46716 and R01HL128831.
Citation: Kant S, Selke FW, Feng J, Usheva A (2022) Why is COVID-19 a Challenge for the Myocardium in Metabolic Syndrome Patients. J Clin Exp Cardiolog.13:706.
Received: 24-Jan-2022, Manuscript No. JCEC-22-706; Editor assigned: 26-Jan-2022, Pre QC No. JCEC-22-706(PQ); Reviewed: 17-Feb-2022, QC No. JCEC-22-706; Revised: 21-Feb-2022, Manuscript No. JCEC-22-706(R); Published: 24-Feb-2022 , DOI: 10.35248/2155-9880.22.13.706
Copyright: © 2022 Kant S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.