Journal of Biomedical Engineering and Medical Devices
Open Access
ISSN: 2475-7586
ISSN: 2475-7586
Research - (2019)Volume 4, Issue 1
Background: Most fMRI studies on pain perception utilized thermal stimuli due to the lack of a non-metallic reliable, reproducible mechanical stimulus. We explored 2 potential MR compatible mechanical devices and validated one that induced changes on fMRI.
Purpose: To validate a noxious mechanical stimulus MR compatible device in a healthy cohort and a chronic pain cohort. Further to ensure this device caused changes on fMRI compared to a pressure device.
Methods: Two-healthy controls underwent fMRI scans in the presence/absence of mechanical noxious stimuli using different devices. A paradigm for administering the stimuli with the most robust responses on fMRI was developed. Reproducibility of the device was confirmed in 10 chronic pain patients and age/gender-matched healthy controls. Comparisons of patient demographics including age, sex, and numerical rating scale (NRS) scores between the cohorts were performed using a two-sample t-test. Reproducibility was assessed through repeated-measures ANOVA.
Results: Both devices, when administered at 15 seconds on, 30 seconds off induced primary somatosensory cortex (SI) activation on fMRI. The device affecting the nail bed induced consistent discomfort and was subsequently validated. Specifically, a mean pressure of 15.7 ± 0.05 psi induced discomfort that remained consistent over repeated trials (p=0.74). Further, in healthy controls, there was a significant correlation between pressure applied and NRS scores in controls(p<0.01). In chronic pain patients, a mean pressure of 15.3 ± 0.10 psi provided discomfort during a single trial. This pressure did not differ than that found in healthy controls (p=0.26).
Conclusion: We have developed and validated a novel pressure device which is MRI compatible and provides for improved accuracy. We have demonstrated increased reproducibility compared to existing devices in literature and documented feasibility in pilot fMRI studies. Future work will focus on demonstrating its use in fMRI studies in chronic pain patients.
Chronic pain; fMRI; Mechanical stimuli; Thermal stimuli; Noxious threshold
Chronic pain is a major cause of morbidity worldwide. The prevalence of chronic pain among adults ranges from 2% to 40% [1]. There is also a major economic burden associated with chronic pain, with the cost of pain management in the US estimated between $560 and $635 billion dollars in 2010 [2]. Unfortunately, the pathophysiology and mechanisms of chronic pain are not well understood, although there is a growing body of evidence being generated through functional magnetic resonance imaging (fMRI) studies.
With fMRI studies, researchers have been able to identify structures within the brain that are associated with the perception of chronic pain. Further, it is possible to determine what brain network changes occur when patients are exposed to noxious stimuli during the fMRI [3]. While it is well documented that different stimuli can illicit different activation sites [4], some stimuli are better understood. Thermal stimuli have been a preferred method for nociception imaging during fMRI because of MR compatibility and ability to control the magnitude and duration of the stimulus. In contrast, mechanical stimuli have been used less frequently in fMRI studies. Additionally, thermal stimuli have demonstrated different neural patterns compared to mechanical stimulus and does not accurately represent the type of pain experienced by chronic pain patients as a mechanical stimulus does [5]. While pressure algometry is a well validated method to reliably produce mechanical stimuli outside the MRI [6], commercial algometers are not compatible with MRI scanners due to ferrous components, limiting how researchers can study mechanical stimulus and pain. The novel device described in this study is composed entirely of MRI compatible components and has a base and finger support to insure consistent pressure (Figure 1). The device was based on the designs of an assessment tool employed in rodent models of chronic pain [7].
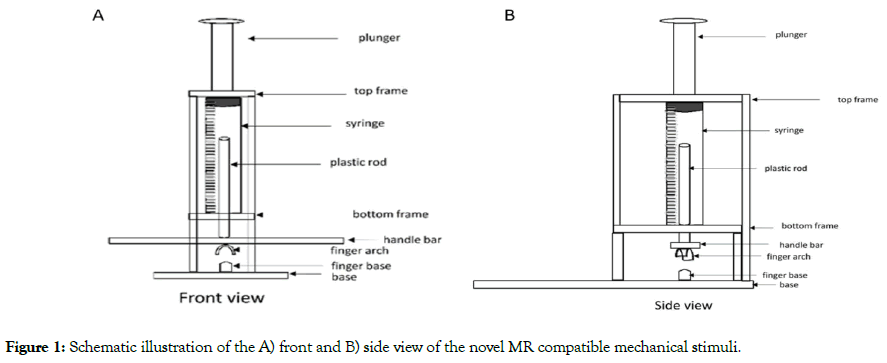
Figure 1: Schematic illustration of the A) front and B) side view of the novel MR compatible mechanical stimuli.
The purpose of this study was to determine whether a mechanical stimulus that was highly reproducible could correlate with numerical rating scale (NRS) scores while in the clinic and the second is to perform a preliminary study to determine if fMRI blood oxygen level dependent (BOLD) changes could be detected in healthy controls. Initial studies focused on two devices to develop an fMRI paradigm and validation studies focused on the device that induced consistent NRS scores. The devices included that shown in Figure 1 and a plastic, elastic pressure cuff placed on the skin immediately distal to the nail bed (Figure 2).
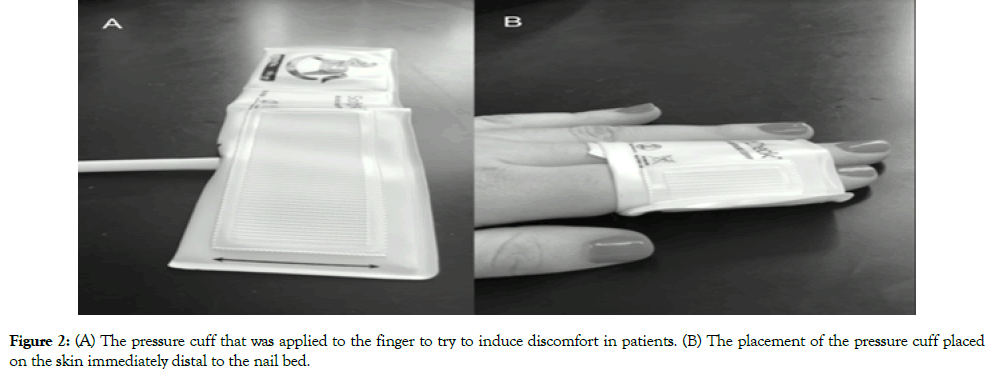
Figure 2: (A) The pressure cuff that was applied to the finger to try to induce discomfort in patients. (B) The placement of the pressure cuff placed on the skin immediately distal to the nail bed.
Subjects
This study was approved by the Institutional Review Board (IRB) and all patients were fully informed upon consent. All participants were over the age of 18, fluent in English, and were able to provide informed consent. Patients were recruited from our center’s Neurology Clinic. Initially, 2 healthy controls with no history of chronic pain underwent fMRI scans in the presence of both devices (Figures 1 and 2). In validation studies, 10 additional volunteers were recruited from the department of neurosurgery, neurology or neuroscience and experimental therapeutics. Controls were considered healthy if they had no presence of chronic pain and no underlying ailments. and Ten patients with chronic pain were recruited from the neurology clinic. Inclusion criteria included presence of chronic pain in limbs or lower back. Exclusion criteria for the chronic pain cohort included those who exhibited chronic migraine related pain.
MRI paradigm
The MRI scans included a high-resolution anatomic 3D T1- weighted FSPGR image acquired for normalization purposes (3T: TR/TE=6.6/2.9 ms, FOV=22 × 22 cm, slice thickness=3 mm, matrix=260 × 260). The fMRI scans were acquired with GE-EPI (3T: FOV=22 × 22 cm, matrix=64 × 64, slice thickness=3 mm, TR/TE=3011/34 ef, bw=250 kHz). All imaging was performed on a 3T MR750 scanner (GE Healthcare, Waukesha, WI, USA). Regions of interest including the primary somatosensory (SI), anterior cingulate cortex (ACC), insula, primary motor cortex (MI), thalamus and prefrontal cortex were analyzed by obtaining the peak voxel tvalue within that brain region. Analysis was performed using Statistical Parametric Mapping (SPM 12, http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and was thresholded at p<0.05 and corrected for multiple comparisons using voxel thresholding. Amount of activation or deactivation was determined from computed t-values.
Pressure device
In healthy controls, we established validity by performing three separate trials with our device on two separate days. The device we designed uses a syringe to apply pressure, which we measured using the gauge on the syringe, with zero representing the pressure at which the device is just touching the finger and providing no discomfort. Measurements were taken in one score increments from initial touch until the patient reached maximum discomfort. The scores obtained were then converted to pressure using P1V1=P2V2 (where P=pressure and V=volume; P1=1 atm).
Then, pressure was applied to induce discomfort. Participants were asked to tell the researcher when they had significant discomfort. The pressure was converted to a score and NRS was recorded. This trial in healthy controls was repeated on a subsequent day for 4 trials in total. In chronic pain patients, a single recording was obtained during a scheduled patient appointment. Due to the availability of our chronic pain patients, subjects were only asked to complete 1 trial. Comparisons of patient demographics including age, sex, and NRS scores between the cohorts were performed using a two-sample t-test. The statistical significance of the pain induced by the mechanical stimulus was calculated by comparing the cohorts using repeated measure ANOVA. All statistical data analysis was performed on IBM SPSS (Version 23, Armonk, NY).
fMRI results
In the initial studies, 2 healthy controls trialed the two devices. Both subjects underwent fMRI with the mechanical stimuli applied for 30 s ON and then 30 s OFF cycling during the 5.5 minute scan. The expected changes in fMRI based on the literature, i.e., SI, ACC, MI, thalamus and insula activation, were not seen with either device (Figure 3A). We altered the algorithm to 15 s ON and 30 s OFF based on further evaluation of the pain stimuli literature [8-11] and found that SI activation was induced with the mechanical device (Figure 3B), further SI deactivation was observed with the pressure cuff. More consistent discomfort over multiple trials was found with the mechanical device (Figure 1) and thus this device was selected for the validation studies.
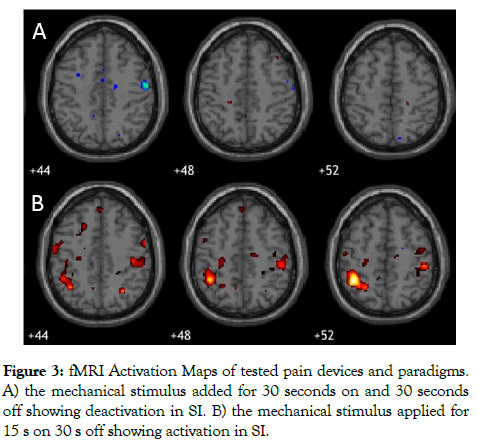
Figure 3: fMRI Activation Maps of tested pain devices and paradigms. A) the mechanical stimulus added for 30 seconds on and 30 seconds off showing deactivation in SI. B) the mechanical stimulus applied for 15 s on 30 s off showing activation in SI.
Validation studies
The validation study group included 10 healthy controls (mean age 40.5, range 27-57) and 10 patients with chronic pain (mean age 49.4, range 23-63) (Table 1). Healthy controls and chronic pain patients did not vary significantly in sex (p=0.67) or age (p=0.10). The chronic pain cohort consisted of patients with Parkinson’s Disease associated pain, chronic neuropathic pain not otherwise specified, migraines, complex regional pain syndrome type 1, diabetic neuropathic pain, and fibromyalgia.
| Characteristics | Cohort | |
|---|---|---|
| Control (n=10) | Pain Patients (n=10) | |
| Age, Mean ± SD (range) (years) | 40.5 ± 10.3 (27-57) | 49.4 ± 11.7 (23-63) |
| Women (%) | 5 (50) | 6 (60) |
Table 1: Characteristics of 20 enrolled patients.
The noxious mechanical stimulus induced discomfort at a mean pressure of 15.7 ± 0.05 psi in control patients. These patients underwent 4 trials with the device over 2 days. Pressure that induced discomfort remained consistent over these repeated trials (p=0.74). Further, NRS scores increased as pressure increased (p<0.01) (Figure 4). In the chronic pain cohort, a mean pressure 15.3 ± 0.10 psi induced discomfort during a single trial. This pressure did not significantly differ than that found in healthy controls (p=0.26). There was no significant difference in NRS scores at highest level of discomfort for healthy controls (mean=5.4 ± 0.7) versus chronic pain patients (mean=4.8 ± 1.4) (p=0.23).
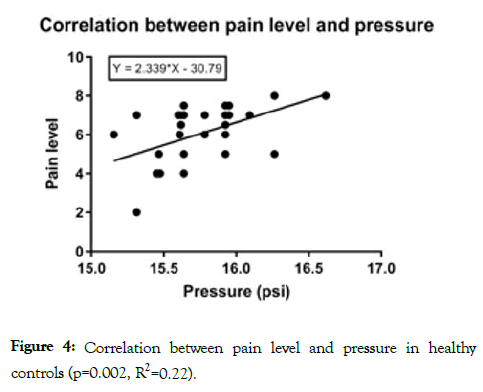
Figure 4: Correlation between pain level and pressure in healthy controls (p=0.002, R2=0.22).
In this study, we demonstrated the validity of an MR compatible noxious mechanical stimuli by establishing the reproducibility of pressure scores necessary to induce pain over 4 trials in healthy controls over two days. There was a significant correlation between pressure scores and NRS scores in healthy controls. A similar pressure induced discomfort in chronic pain patients in a single trial though no correlation with NRS scores were found.
The experiments to validate our novel device as well as to evaluate differences in pain thresholds began with determining whether devices could induce changes on fMRI. We specifically developed our stimuli algorithm based on modifications of methods that have been reported in literature [8-11]. It appears essential to allow a minimum of 20-30s between stimuli [8-11]. However, the duration of stimuli largely varies depending on the instrument selected to provide mechanical pain. In one study which used von Frey filaments to induce pain, a paradigm that consists of 6s on and 30s off was implemented [8]. Long stimulation can cause BOLD signals to return to baseline as patients become accustomed to the pain [12] while shorter stimulation periods prevent this. However, the nature of our device currently requires researchers to steadily press onto the syringe, which limits our ability to apply the stimuli in a short period of time.
We have thus shortened our pain paradigm to the extent that the accuracy of applied pressure is ensured. Our fMRI results show that our 15s on and 30s off paradigm with our mechanical pain stimulus produced activation in brain regions associated with defined pain pathways including SI. We have previously tested a mechanical stimulation device consisting of a blood pressure cuff placed around the index finger but found that the device did not induce discomfort in healthy controls. Our novel stimulation device induced pain consistently in healthy controls; additionally, less time is needed to depress a plunger than to inflate the cuff, which is preferable in the MR environment. The pressure cuff applied for the same paradigm produced the opposite effect in SI showing deactivation instead of activation. Previous findings suggests that applied pressure will induce deactivation in SI, MI and ACC, however when pressure turns to discomfort SI, MI and ACC will become activated [11] which is consistent with our findings.
The majority of previous fMRI studies have used thermal over mechanical stimuli, due to ease of use in the fMRI, resulting in a lack of validated noxious mechanical stimuli established for use with fMRI. A meta-analysis demonstrated differences in neural patterns depending on the type of stimuli employed [5]. Briefly, thermal stimuli have been shown to activate the prefrontal cortex (PFC), right insula, cerebellum, thalamus, amygdala, hypothalamus and putamen [13,14]. Mechanical stimuli demonstrated activation in the SI, premotor and supplementary motor regions, dorsal anterior cingulate cortex (DACC), caudate and striatum [4,15-17]. As noxious mechanical stimuli and noxious thermal stimuli have been demonstrated to activate separate neural pathways, findings as to brain regions involved in the dimensions of pain in thermal fMRI studies cannot be effectively translated to mechanical ones without further study.
Use of noxious mechanical stimuli with fMRI is limited to three studies, all of which induced pressure with the same modified algometer [15-17]. One found a lower pain threshold and a greater affective reaction to pain, as measured by the visual analogue scale (VAS), in chronic pain patients over healthy controls, consistent with our results [16]. Although the studies made headway in establishing a valid mechanical stimulus device, the modified algometer used did not allow for consistent pressure and did not restrict movement. The base for our device allows for consistent pressure, regardless of body habitus, while our finger support restricts movement [18-20]. Thus, our novel noxious mechanical stimulation device improves upon those existing in the literature.
Validating a noxious mechanical stimulus device would further provide an invaluable tool in improving our understanding of pain pathways as mechanical stimulus induces a more accurate representation of pain seen in chronic pain patients. For instance, musculoskeletal pain has been the most commonly characterized pain that disturbs PD patients with a prevalence ranging from 45% to 74% [21,22]. Therefore, the advantages of using a noxious mechanical stimulus device lie in its generation of somatic pain that better approximate the muscle soreness-like sensation in patients [23]. In addition, establishing an effective mechanical stimulation device would allow for comparison between pressure delivered and pain perceived, expanding our understanding of the subjective nature of chronic pain.
Our study, however, necessitates future exploration. NRS scores are a subjective measure and could be why there was no significant difference in NRS scores between healthy and pain patients. We further acknowledge our device has limitations. As the device is not automated, an additional person must be involved in order to operate the device., This produces variability in time for the human operator to depress the plunger, resulting in inconsistencies. Therefore, modification of the device to advance the plunger remotely would be of great benefit. Moreover, using a syringe with a smaller diameter, which increases the distance between start and end point of measurement, may help in more precise readings of the numerical rating scale. Further, by providing a pressure meter to the syringe would allow for more accurate recordings and would greatly enhance the device. We acknowledge that our subcohort of healthy controls who received an fMRI scan was small and should be considered a limitation of this study. For this reason, fMRI results should be considered preliminary and further investigation using our mechanical stimulus to induce fMRI changes is needed.
The present study demonstrated that our device administered at 15s on and 30s off induced SI patterns consistent with application of mechanical stimulus. Further we have demonstrated validity and reproducibility in both chronic pain and healthy controls. We hope to expand on the current pain study methodology and provide opportunities for more comprehensive investigations on possible altered pain perception in chronic pain patients in the future.
Dr. Pilitsis is a consultant for Boston Scientific, Nevro, Jazz Pharmaceuticals, and Abbott and receives grant support from Medtronic, Boston Scientific, Abbott, Nevro, Jazz Pharmaceuticals, GE Global Research, SBIR 1R43NS107076-01A1, and NIH 2R01CA166379-06. She is medical advisor for Aim Medical Robotics and Karuna and has stock equity. Ileana Hancu is an employee of NIH and a former employee of GE global research. Eric Fiveland is an employee of GE global research.
Citation: DiMarzio M, Spinrad M, Hancu M, Grinde E, Chang J, Slyer J, et al. (2019) Validation of a Novel Noxious Pressure Device for Use in fMRI Pain Studies. J Biomed Eng Med Devic 4:138. doi: 10.35248/2475-7586.19.4.138
Received: 08-Apr-2019 Accepted: 24-Apr-2019 Published: 29-Apr-2019 , DOI: 10.35248/2475-7586.19.4.138
Copyright: © 2019 DiMarzio M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : Medtronic, Boston Scientific, Abbott, Nevro, Jazz Pharmaceuticals, GE Global Research, SBIR 1R43NS107076-01A1, and NIH 2R01CA166379-06