Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2021)
Background: Pre-Exposure Prophylaxis (PrEP) is integral to the US End of AIDS strategy. However, low adherence, high costs, frequent testing and monitoring side effects make delivery of PrEP complicated. Gilead has sponsored PrEP-related research and access as part of its marketing efforts. We review Conflict of Interests (COI) in the scientific literature for the US PrEP-related articles to understand the potential impact of Gilead’s corporate sponsorship.
Methods: We searched PubMed for US PrEP articles published in 2018 in the top 10 medical journals and top 10 HIV/AIDS journals and abstracted information on author/institutional COI, type of COI, and favorability of results and conclusions. We identified first three and senior authors from the articles and the leading institutions, defined as institution of three or more authors or participating institutions in a trial. We conducted searches onGoogle, PubMed, ClinicalTrials.gov, OpenPaymentsData.cms.gov, others to identify potential Gilead support to authors and institutions.
Findings: Our search identified 93 articles. Of the 289 first three and senior authors in these articles, 34(11%) declared a Gilead COI and 28(10%) had undeclared Gilead COI. Authors were from 51 leading institutions, of which, 12(24%) declared Gilead COI and 22(45%) had undeclared COI. Overall, 30(32%) of the 93 articles had declared Gilead COI. Combining declared and undeclared COIs for authors and institutions provided an overall 83(89%) articles with a potential Gilead COI. Declared Gilead support was significantly associated with favorable conclusions (p<0.05); combined declared and undeclared author and institutional Gilead support was not.
Discussion: Nearly 90% of US PrEP articles had Gilead support and authors failed to report COI in 70% articles. Direct corporate support is important for scientific research. However, Gilead’s marketing push for PrEP, undeclared COI, and influence of Gilead supported authors are concerning given the potential impact on US’s HIV control strategy.
HIV; COI; Pre-exposure prophylaxis; AIDS
After over 40 years, the HIV pandemic remains a serious threat for millions of people worldwide [1]. In 1996, the discovery of effective HIV treatment changed the response, including further studies showing that treatment prevents illness, death and reduces the risk of transmission by 100% [2-7]. Although treatment and other prevention interventions are now available in the United States, in 2019, there were around 1.2 million people living with HIV and 35,000 people were newly infected [8]. Current treatment strategies have improved the quality of life for those living with HIV including the potential to live a near normal lifespan [9]. However, around 520,000 people were still not virally suppressed and there were 16,000 people who died from complications associated with HIV in 2019 [8]. As with the COVID 19 pandemic, the domestic HIV epidemic disproportionately impacts vulnerable people including poorer people and Black, Indigenous, and People Of Color (BIPOC) [8]. Despite considerable investment, after 40 years, it is still unknown when a HIV cure or vaccine will become available [10,11]. Treatment has had a major impact on the pandemic and has decreased illness, death and transmission in many settings [2-7]. As the most effective prevention intervention, when combined with other forms of prevention (e.g., condom use, male circumcision, pre-exposure prophylaxis, and harm reduction for drug users) and social support, treatment has the potential to eliminate HIV [12,13].
In response to the ongoing HIV epidemic, in 2019 the Trump administration announced the launch of Ending the HIV Epidemic: A Plan for America (EHE) during the State of the Union Address [14,15]. The objective of EHE is to reduce the number of newly diagnosed individuals by 75% within 5 years and by 95% within 10 years. In order to meet this goal, the US Department of Health and Human Services (HHS) will focus on 4 key strategies: 1) Ensure early diagnosis of infection; 2) Treat HIV rapidly and effectively to achieve sustained viral suppression; 3) Prevent at- risk individuals from acquiring HIV infection, including the use of Pre-Exposure Prophylaxis (PrEP); and 4) Rapidly detect and respond to emerging clusters of HIV infection to further reduce new transmission. Under the Biden administration, the Ending the HIV Epidemic plan is still in place and serves as the basis for current US efforts to address HIV [16].
At the center of EHE is the administration of PrEP to those individuals who are considered to be at risk of contracting HIV. Emtricitabine/tenofovir disoproxil received US Food and Drug Administration (FDA) approval for PrEP, after the iPreX and Partners PrEP trials supported the efficacy and relative safety of the medication in men who have sex with men [17-19]. Some studies indicate that PrEP can reduce the risk of sexually transmitted HIV infections by 99% by taking a daily combination drug composed of emtricitabine/tenofovir disoproxil [20-22]. Despite these promising results, a number of trials have also shown zero impact with low adherence posing a major challenge, particularly for women [23-25]. Delivery and adherence to PrEP is also complicated as it requires people to identify as being at risk for HIV acquisition, frequently be tested for HIV, and monitoring for potential side effects [26,27].
Despite significant investment in efforts to promote PrEP, it has been controversial and so is its role in national HIV responses. Specifically, the emphasis on PrEP can be seen to be competing with access to early diagnosis and treatment, particularly in places with scarce resources and limited access to health care [28,29]. Media and others sometimes confuse PrEP with treatment, the importance of access to treatment is often not stated, and it is often compared as an equally important intervention as access to treatment [30,31]. PrEP is expensive and in the US costs around $ 20,000 per year with Gilead, the manufacturer of emtricitabine/ tenofovir disoproxil, reportedly garnering over US$ 3 billion in Truvada sales in 2018 and over US$ 36 billion since 2004 [32]. Initial Gilead support for expanding the use of Truvada in the US has now been scaled back, threatening the existence of many clinics providing PrEP to vulnerable populations [33].
The introduction of a new and more expensive new regimen also poses economic challenges for small clinics while increasing Gilead profits [33]. Delivering PrEP overseas is also costly and requires additional testing and monitoring for people to ensure that they do not become HIV positive and/or do not develop side effects. Gilead has been embroiled in a number of lawsuits, including a patent dispute with HHS and claims that it purposefully delayed the introduction of a safer version of PrEP that relies on their new drug in order to increase profits [34,35].
Corporate influence through sophisticated marketing strategies that include direct and indirect financial support is increasingly recognized as a serious threat to scientific discourse, regulatory standards, and policy formulation [36]. Gilead, as part of its core business model, is a major sponsor of PrEP related research efforts, institutions, community organizations, meetings, and scientists. To the best of our knowledge, there has not been an effort to review and analyze the impact of Gilead’s corporate sponsorship on the scientific literature and discourse. In an effort to better understand the impact of corporate influence on the US national scientific discussion and HIV response, we review potential conflict of interests in the scientific literature for domestic PrEP related articles.
This study was not subject to institutional review board oversight as it did not meet the regulatory definition of human subject research per federal regulations.
Search strategy
In May 2021, we searched PubMed for all articles containing the keywords HIV or AIDS or Human Immunodeficiency Virus or Acquired Immunodeficiency Syndrome and PrEP or Pre- Exposure Prophylaxis or Truvada or HIV prophylaxis. We included commentaries, reviews, case reports and research papers on HIV prophylaxis in humans published from 1/1/2018 to 12/31/2018. The year 2018 was chosen as it coincided with formulation of the US national HIV strategy under the Trump Administration. We selected articles from the top 10 medical journals (excluding HIV/AIDS specialty journals) and the top 10 HIV/AIDS journals as determined by their h5-index [37,38]. We then excluded all articles that were not based in the US or focused on basic science physiology (Figure 1).
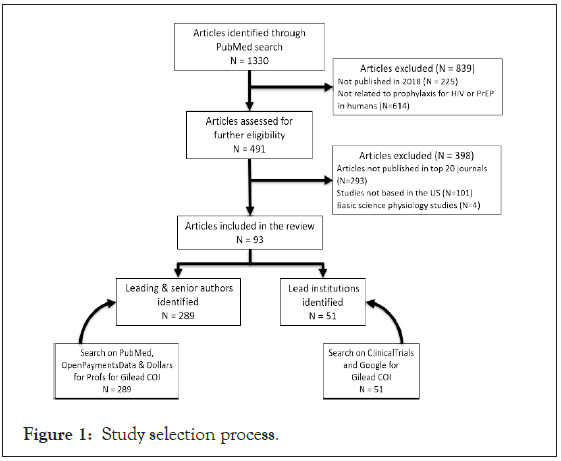
Figure 1: Study selection process.
Literature review
We reviewed the included articles for the following information: authors, type of article, journal, leading institution(s), declared Conflicts Of Interest (COI), type of Gilead support (if any) to authors and institutions, favorable vs. non-favorable results for PrEP as an intervention in the results, and favorable vs. non- favorable conclusion for PrEP as an intervention or further research in the conclusion. The results were considered favorable if only positive results were reported for all study populations and unfavorable if negative or mixed results were reported for one or more of the populations. The conclusion was considered favorable if the discussion drug or intervention was recommended for scale- up and unfavorable if the conclusion section directly stated that the pharmacologic therapy was not superior or contained reservations about PrEP. Gilead support was defined as the author and the institute receiving grant, funding, fees, non-financial support, study drugs and/or reimbursements for food, travel and meetings.
We then selected the first three authors, the last author, and any authors who declared a COI with Gilead to determine if they had undisclosed Gilead support. We used two different search strategies: 1) PubMed search for the “author’s name” and “Gilead”. We extracted all declared COI information from the authors’ manuscripts published from 1/1/15-12/31/18 (articles dating back to 2015 were included because the ICMJE recommends disclosing all revenue paid for 36 months prior to submission) and 2) we searched “author’s name” in the public databases OpenPaymentsData.cms.gov and ProPublica’s Dollars for Profs [39-41].
OpenPaymentsData.cms.gov is a transparency program that collects and publishes financial relationships between the device or drug companies and physicians. Since it is geared toward identifying relationships between physicians and the healthcare industry, we also searched Dollars for Profs to include any professors, researchers, or staff not identified by OpenPaymentsData.cms.gov.
We selected the leading institution(s) for an article as (1) the affiliated institution(s) of three or more of the authors or (2) the participating institution(s) in a trial as reported by ClinicalTrials.gov [42]. Otherwise, the institution of the first author was selected as the lead institution. We searched ClinicalTrials.gov using the keywords “HIV (Condition or Disease)”, “PrEP, Gilead, Institution name (Other terms)” and “United States (Country)” for Gilead support (grant, funding or collaboration) to an institution. Secondly, we searched Google with the “Institution name” and “Gilead” to determine if there was any support reported on the Gilead’s or institution’s website between 2015-2018.
Statistical analysis
Using chi-square distribution, we examined whether favorable conclusions were associated with a) declared Gilead COI by authors and institutions and b) both declared and undeclared Gilead COI by authors and institutions. The analysis was performed on Social Sciences Statistics [43].
Literature search results
Among 1330 manuscripts identified, 93 were in the top 20 journals and met the inclusion criteria as described in the methods section (Figure 1). The majority of the articles were published in three of the 20 top journals-28 (30%) in AIDS and Behavior, 24 (26%) in Journal of Acquired Immune Deficiency Syndromes (JAIDS) and 11 (12%) in PloS One.
Of the 93 articles, 69 (74%) were research articles, nine (10%) articles were reports and reviews, eight (8%) were letters to the editors and case reports, and seven (8%) were commentaries or viewpoints. For the 69 research articles, conclusions were favorable for 50 (72%) studies, unfavorable for two (3%) and remaining 17 (25%) concluded ‘further research’. For nine reports and reviews, conclusions were favorable for seven (78%) and two (22%) concluded ‘further research’. In the eight articles that were case reports and letters, no conclusions were made. For seven commentaries/viewpoints, conclusions were favorable in three (43%), unfavorable in one (14%) and three (43%) concluded ‘further research’. In total, there were 85 articles with outcomes, with favorable conclusions in 60 (71%), unfavorable in three (4%) and ‘further research’ needed in 22 (25%). Of the 85 articles, Gilead COI (by author and/or institution) was declared in 26 (31%). Declared author and institutional Gilead support was significantly associated with favorable article conclusions (X2(1, N=85)=5.2, p=0.02 (p<.05)).
Authors declared and undeclared conflicts of interest
From the 93 articles, there were 289 first three and senior authors. Of the 289 authors, 34 (11%) declared a Gilead COI. The secondary search for potential Gilead COI found an additional 28 (10%) authors with COIs for an overall 62 (21%) authors with declared and undeclared Gilead COIs (Figure 2).
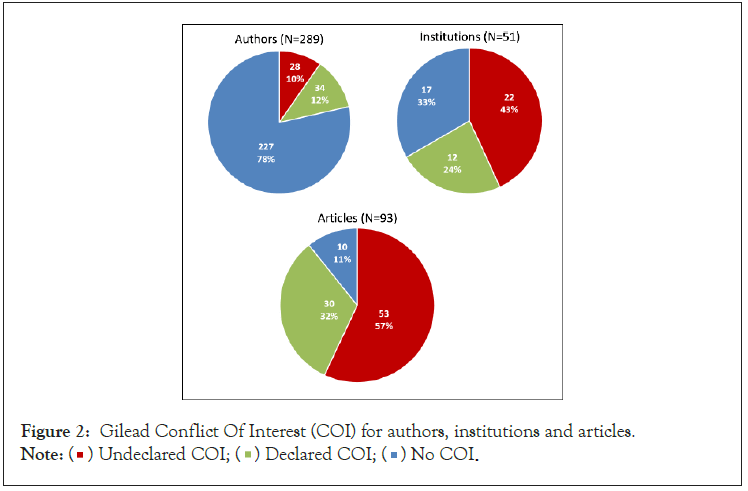
Figure 2: Gilead Conflict Of Interest (COI) for authors, institutions and articles.
Note:  No COI.
No COI.
Between 2015-2018, the authors received multiple types of support from Gilead. Of the 62 authors with declared and undeclared Gilead COIs, 33 (53%) received grants and/or research funding; 23 (37%) received consultancy fee and/or honorarium, 17 (27%) received drugs for studies and 18 (29%) authors received reimbursements for travel, lodging and food. Other types of support ranged from contracts, personal fee, and fee for members/ participants at Gilead’s Scientific Advisory Board meetings, and support for speaking, educating and training. There were nine (15%) of 62 authors who received only study drugs from Gilead, sometimes along with other non-financial support (drug level testing, administrative or equipment).
Of the 289 authors reviewed, ten (3%) published five or more article on PrEP in 2018. Of these ten authors, seven (70%) had declared and undeclared COIs. They were co-authors on 46 (49%) of the 93 selected articles (Table 1).
| Author number | Declared or undeclared COI | Type of Gilead support | |||||
|---|---|---|---|---|---|---|---|
| PrEP articles published in 2018 (%) | Grant/ research funding | Drugs for study | Consultancy fee | Travel, lodging and food | Others | ||
| 1 | Yes | 11(12%) | Yes | No | Yes | Yes | Unrestricted project support |
| 2 | Yes | 9(10%) | Yes | Yes | No | Yes | Unrestricted funds to employees |
| 3 | Yes | 8(9%) | Yes | No | No | Yes | No |
| 4 | Yes | 7(8%) | Yes | No | Yes | No | Scientific advisory board expert |
| 5 | Yes | 6(6%) | No | Yes | No | No | Support for drug level testing |
| 6 | Yes | 6(6%) | No | No | No | No | Scientific advisory board member |
| 7 | Yes | 5(5%) | No | No | No | No | No |
| 8 | No | 5(5%) | Yes | Yes | No | No | Support for DBS testing |
| 9 | No | 5(5%) | No | No | No | No | No |
| 10 | No | 5(5%) | No | No | No | No | No |
| Total | 7(70%) | 46(49%) | 5(50%) | 3(30%) | 2(20%) | 3(30%) | |
*No signifies negative result on search.
Table 1: Gilead support for top 10 authors with five or more publications in 2018.
Institutions declared and undeclared COI
The 93 publications were associated with 51 leading institutions (see methods for definition). Of these institutions, 39 (76%) were universities. Of the 51 institutions, authors from 12 (24%) declared an institutional Gilead COI including grants, funding, research support and/or contract. After searching ClinicalTrials.gov and Google, we found an additional 22 (45%) undeclared institutional support for an overall 34 (69%) institutions with a potential Gilead COI (Figure 2).
Overall declared and undeclared COI
Of the 93 included articles, 29 (31%) included a declared Gilead COI by at least one author and 33 (36%) had undeclared Gilead support for one or more authors. Of the 93 articles, 17 (18%) had declared and 59 (63%) had undeclared institutional support (Table 2). There was a declared author and/or institutional Gilead COI in 30 (32%) of the 93 articles (Figure 2).
| Authors | |||||
|---|---|---|---|---|---|
| Institutions | Declared | Undeclared | No COI | Total | |
| Declared | 16(17%) | 1(1%) | 0(0)% | 17(18%) | |
| Undeclared | 10(11%) | 28(30%) | 21(23%) | 59(64%) | |
| No COI | 3(3%) | 4(4%) | 10(11%) | 17(18%) | |
| Total | 29(31%) | 33(35%) | 31(34%) | 93(100%) | |
*COI: Conflict Of Interest.
Table 2: Author and institutional COI in the 93 articles.
Combining author declared and undeclared COIs with the declared and undeclared institutional COIs provided an overall 83 (89%) articles with a potential author and/or institutional conflict of interest. Combined declared and undeclared author and institutional support were not associated with a favorable conclusion according to the chi-square test.
Direct corporate support is an accepted element of modern scientific research. However, considerable efforts have been made to ensure that consumers of scientific articles are made aware of authors’ potential conflicts of interest. Disclosure and recognition of potential conflicts are particularly important when evaluating articles for publication and during clinical and public health policy formulation. Gilead reported earnings of US$ 3 billion from Truvada sales in 2018 and its annual report suggested that US$ 138 million was devoted to marketing efforts between 2012- 2018 [32]. Our research found that 89% of the United States 2018 PrEP articles published in the top 20 journals had declared and undeclared potential conflicts of interest due to support from Gilead, the manufacturer of the recommended PrEP regimen. The authors from 64 (69%) articles we reviewed failed to report potential individual and/or institutional conflicts of interest as part of their article submission. Gilead provides funding for many institutions and only nine authors made the connection and mentioned potential institutional conflict of interest in their declarations. Although it is often possible to determine institutional support through relatively simple internet searches, whether the support is part of an authors’ compensation package and/or otherwise impacts their research efforts and conclusions is difficult to determine.
Only ten authors accounted for around 50% of the articles we reviewed with 70% of these authors having potential conflicts of interests including receiving grants, speaker fees, institutional support, and study drugs. This disproportionate author impact on the PrEP literature combined with conflicts of interest may have had undue influence on the scientific discourse around the science around PrEP and its role as part of the US EHE. While difficult to prove with certainty, publishing positive articles regarding PrEP may have influenced editors and scientists to frame research questions positively and draw similar conclusions from the data.
PrEP has been showed to be effective, however there are a number of challenges to its effectiveness as a HIV control intervention including acceptance, adherence, its $20,000 a year cost and the need for complicated long-term monitoring including frequent HIV testing [26,27,32]. Declared conflicts of interest due to Gilead support were significantly associated with positive conclusions for the articles with many of the articles minimizing or not discussing the efficacy data of PrEP from different trials and challenges associated with scaling PrEP as a means of HIV control. PrEP is central to the US EHE as the priority prevention intervention necessary for US HIV control and considerable resources and efforts have been mobilized to deliver it to the millions of people who are at risk of HIV infection [15]. From 2019, Gilead donated Truvada-based PrEP to 200,000 uninsured people annually, however, the US government is required to cover delivery costs and some have argued that this is part of building a market for the newer more expensive regimen [44].
Ironically, the potential US market for PrEP is directly dependent on the number of people who are already infected with HIV and who are not on successful HIV treatment-the more people who are not virally suppressed, the higher the risk of ongoing HIV transmission and the more necessary are other prevention interventions such as PrEP. Successful HIV treatment is the most effective available tool as it prevents illness, death and 100% of transmission. Successful treatment for the UNAIDS 90-90-90 target (73% of people living with HIV virally suppressed) has already been achieved in many other countries in Europe and Africa and urgently needs to be made the highest priority in the US where 43% or over 520,000 people are still not on successful treatment [8]. A policy discussion free of profit motive should consider the role of PrEP and other prevention interventions in the context of achieving universal access to testing, treatment and care. Removing the profit motive from scientific and strategic discussions is increasingly important as it may lead to prioritizing treatment access first-this is important in the US where hundreds of thousands of people are not yet on successful treatment. Policy discussions focused on the role of PrEP in low-and middle-income countries are particularly vulnerable to financial conflicts of interest as access to treatment and care for over 10 million of people living with HIV is extremely limited [45].
Our study had a number of limitations. Although we limited our focus to a single year, the first three and senior authors, US focused articles, and the top 20 journals, this allowed us to conduct a more in-depth search for declared and undeclared conflicts of interest. While all of the articles had conflict of interest declarations, our secondary search strategy showed that many of the conflicts are not declared. Determining Gilead support for institutions relied on declared support and it is likely that some support was not available through database searches. Additionally, some of the smaller potential conflicts such as free meals, meeting expenses, and other inducements may not be in the public domain. Therefore, it is possible that we missed conflicts for some authors and institutions making our findings more conservative. A potential conflict of interest does not automatically translate into poor science and association with a favorable article is not causation. Just because much of the scientific content has declared and undeclared conflicts of interest it does not mean that it is not valid nor does it necessarily lead to a lack of prioritization of other interventions. This is particularly true when large numbers of articles and experts, many without potential conflicts, are consulted to formulate national HIV control policy. However, regardless of the nature of the association and the likelihood of a causal effect on final policy formulation, our review suggests that disclosures and awareness of potential conflicts could be improved. Despite the limitations, our data suggests that lack of disclosure and awareness of conflicts have the potential for serious consequences for national HIV control strategies as the selection and prioritization of interventions do influence individual and population-level outcomes.
Our rapid review of potential conflicts of interest due to Gilead support showed that a majority of the US 2018 scientific articles on PrEP published in the 20 top journals had received some form of Gilead support. Additionally, a large proportion of the conflicts of interest were not declared. We focused on the United States, leaving open the question of Gilead support for PrEP studies in low-and middle-income countries with limited HIV resources, especially where trials are led by academic institutions in the US. Although PrEP has been proven to prevent HIV acquisition, the important and ongoing corporate marketing push for PrEP versus other interventions and the potential undue influence of a few Gilead supported authors is of concern. Corporate support of research is important to advance science; however, hidden or undeclared conflicts of interest can compromise clinical and national policy recommendations regarding HIV prevention. Most potential conflicts are already in the public domain and authors, editors, and policy makers should be more careful to declare conflicts so that consumers of science can be fully aware of potential biases when considering the science and experts’ conclusions.
Citation: Gupta S, Khan S, Vaheed R, Granich R (2021) United States Ending the Human Immunodeficiency Virus (HIV) Epidemic Plan: Evaluation of the Role of Industry Funding in Published Pre-Exposure Prophylaxis (PrEP) Literature. J Antivir Antiretrovir. S22:001.
Received: 07-Oct-2021 Accepted: 21-Oct-2021 Published: 28-Oct-2021 , DOI: 10.35248/1948-5964.21.s22.001
Copyright: © 2021 Gupta S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.