Clinical Pediatrics: Open Access
Open Access
ISSN: 2572-0775
ISSN: 2572-0775
Research Article - (2023)Volume 8, Issue 1
Background: Treatment outcome results serve as a proxy of the quality of TB treatment for children in Ethiopia; data on TB treatment outcomes in children are limited. The aim of this study was, therefore, to determine the magnitude of tuberculosis treatment outcomes in children and associated factors in health facilities of Shashemene, Southern Ethiopia.
Methods: A cross-sectional study design was conducted from January 2016 and December 2021, at Shashemene town, Southern Ethiopia. Using a simple random sampling method, 390 registered TB patients who had known treatment outcomes were selected from the unit TB register logbook. The data was entered to Epi Info version 7 and analyzed using SPSS version 23 the characteristics and the treatment outcomes of patients were summarized using descriptive statistics.
Significant variables at p-value <0.25 in the bivariate analysis were entered to multivariable logistic regression. Multivariate logistic regression model was used to find factors associated with tuberculosis treatment outcomes in children.
Results: Out of 390 children, 201 (51.5%) were females, 138 (35.4%) were under 5 years old, and 373 (95.6%) of them were new cases. Pulmonary smear-negative TB accounted for more than half 213 (54.6%), EPTB accounted for 140 (35.9%) and pulmonary smear-positive TB accounted for 37 (9.5%). The overall treatment success rate was 356 (91.3%). Among 390 patients, 25 (6.4%) were cured, 331 (84.9%) were treatment completed, 14 (3.6%) were lost to follow up, 17 (4.4%) were deaths, and 3 (0.7%) were treatment failures. Age group 5 years-9 years (AOR=0.362, 95% CI (0.138-0.950), 10 years-14 years (AOR=0.354, 95% CI (0.130-0.963), lost to follow up category of TB (AOR=8.166, 95% CI (1.437-46.410), HIV positive sero-status (AOR=5.822, 95% CI (2.009-16.869), and rural residence (AOR=2.390, 95% CI (1.002-5.702) were independently associated with treatment outcomes.
Conclusions: The treatment success rate was above the end TB strategy. The treatment outcome was considerably varied with age, HIV status, and residence of the patient. Young children less than 5 years, HIV co-infected, and those patients with rural residence needs follow-up to reduce poor treatment outcomes among children.
TB; Children; Treatment outcome; Shashemene; Ethiopia
Tuberculosis is one of the top 10 causes of mortality, and the leading cause of a single infectious agent (above HIV/AIDS); millions of people continue to fall sick with the disease each year [1]. Worldwide, tuberculosis is one of the leading causes of morbidity and mortality among infectious diseases with 90% of patients living in developing countries, specifically sub-Saharan African countries [2]. Worldwide, 1.3 million children develop active TB every year and 450,000 children die [3]. Between 2000 and 2020, nearly 1 billion people will be newly infected with tuberculosis, 200 million people will develop the disease, and 35 million will die from TB [4]. In children, TB control programs are important to achieve and sustain acceptable levels of treatment success among all TB patients. Therefore, treatment success is measured by a standardized process of treatment outcome monitoring [5]. Treatment success has been measured by the number of patients being cured and those having their treatment completed [6]. Successful treatment of TB in children has obvious benefits to both individual patients as well as the community. Hence, it can have an immediate impact on TB prevalence and mortality rates [7]. The treatment success rate among all new TB cases was 86% globally. This proportion varied from 75% in the European Region to 92% in the Western Pacific Region among the six World Health Organization (WHO) regions [8]. A systematic review in European Union countries also showed that treatment success rates varied from 60% to 87%. Some factors, such as the high prevalence of HIV/AIDS, high prevalence of drug resistance, poor quality of medical services and delay in detection affect the treatment success in children [9].
Treatment outcomes in children for TB disease in the African region have shown high rates of poor outcomes (deaths, treatment failures, and Lost To Follow-Up [LTFU]) ranging from 10% to 19% [10,11]. In Ethiopia, little is known about the epidemiology, prevalence and treatment outcomes of tuberculosis in children. The contribution of childhood TB, as well as its treatment outcomes, is not well documented. Similar to other high-burden countries, Ethiopia faces challenges in capturing childhood TB cases to be treated under the national tuberculosis control program. The lack of such information makes monitoring and evaluating control efforts difficult. Efforts to reach the global target of 90% treatment success, it is important to identify, describe, and deal with factors determining poor treatment outcomes in children. Several reasons and factors for unsuccessful TB treatment outcomes in children have been reported from different countries [12-15]. However, up to the research’s awareness, it is not clear which factors are major contributors to the unsuccessful TB treatment outcomes in children of TB patients in Shashemene town, Oromia regional state, Southern Ethiopia. The aim of this study was, therefore, to determine the magnitude of tuberculosis treatment outcomes in children and associated factors in health facilities of Shashemene, Southern Ethiopia.
Study area
The study was conducted in Shashemene town, West Arsi zone, Oromia Regional State, Ethiopia which is located 250 kilometers to the South of the capital city, Addis Ababa, situated at a crossroad to Bale, Arsi, Zeway, Hawassa, and most parts of Southern Ethiopia. Shashemene town is a separate woreda in West Arsi Zone, Oromia Region. It is the capital city of West Arsi Zone and is located on the main road from Addis Ababa to Hawassa. It has latitude of 7°12' north and a longitude of 38° 36' east [16]. As per Shashemene town health office statistics, the projected total population of Shashemene town was about 264,780. Out of which 131,952 were males and 132,828 were females. About 126,036 were children for the year 2021. Shashemene town has 10 kebeles [17]. Regarding the health institutions in Shashemene town, there are 3 hospitals (2 governmental and 1 private), 5 health centers (4 governmental and 1 private health center), and 20 private clinics. Among these health facilities, 3 hospitals, 4 health centers, and 2 private clinics were offering tuberculosis diagnostic and treatment services for more than six years to the population of Shashemene town and the surroundings. The DOTS (Directly Observed Treatment Short-course) clinic at the hospitals and health centers operates under the National TB and Leprosy Program (NTLCP) of Ethiopia [18].
Study design and study period
Facility based cross-sectional study design was used. The study was conducted from February 10 to March 10, 2021.
Source population and study population
All children less than 15 years of age who were treated for TB under the DOTS program between January 2016 and December 2021 at the health facilities of Shashemene town were the source population. They were children diagnosed with any form of TB and whose treatment outcomes were evaluated between January 2016 and December 2021.
Inclusion criteria
All children with tuberculosis who were registered on the unit TB register logbook in selected health institutions from January 2016 and December 2021.
Exclusion criteria
Those children with tuberculosis whose treatment outcomes were not evaluated/registered, who were transferred out to other health facilities and who were on anti-TB treatment and didn’t complete their treatment during the study period were excluded from the study.
Sample size determination
To determine the sample size for tuberculosis treatment outcomes, the single population proportion formula was used.
The treatment success prevalence of 85% was taken from a previous study conducted in Addis Ababa. The formula below with 5% marginal error and 95% CI were used:
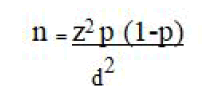
Where, d2=margin of error which is 5%
z=standard normal distribution corresponding to significance level at α=0.05 which was 95%=1.96
p=the proportion which was 85%
n=sample size
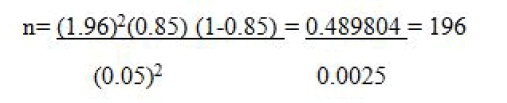
Even though the sample size was 196 there were incomplete data; so, the data incompleteness in this study was estimated to be 10% i.e.
Incomplete data=196*10%=196*10/100=19.6≈20
nf=196+20=216
Hence the overall sample size was 216.
However, to determine the factors associated with tuberculosis treatment outcomes, double population proportion formula with the following assumptions was used. Type one error of 5%, power of 80%, and the ratio of exposed to non-exposed 1:2 and by taking odds ratio and percent of outcome in the unexposed group from previous studies. By substituting the above assumptions into epi info version 7 software stat calc programs, and summarized in the Table 1 below 1.
| Variables | CI (%) | OR | Ratio | Power (%) | Percent (%) of outcome in the unexposed group | Total sample size |
|---|---|---|---|---|---|---|
| Age | 95 | 2.7 | 01:01 | 80 | 48.8 | 140 |
| 10-14 years (unexposed); 0-4 years (exposed) |
||||||
| Residence | 95 | 0.05 | 01:01 | 80 | 53.1 | 30 |
| Urban (unexposed); Rural (exposed) |
||||||
| Type of TB | 95 | 0.47 | 01:01 | 80 | 83.1 | 320 |
| SNPTB (unexposed); SPPTB (exposed) |
||||||
| HIV status | 95 | 0.49 | 01:02 | 80 | 79.9 | 354 |
| Negative (unexposed); Positive (exposed) |
Table 1: Sample size determination for factors associated with tuberculosis treatment outcomes.
Among variables, HIV status was taken as the main exposure variable for TB treatment outcomes in children during 6 months follow-up period since it was considered to give an optimal sample size and the most significant result. In this regard, the total minimum required sample size became 354 with a 10% contingency modifier it became 390.
Sampling technique and sampling procedure
In Shashemene town, there were 3 hospitals, 4 health centers, and 2 private clinics which gave DOTs services for more than 6 years. Populations living around these hospitals, health centers, and private clinics were assumed more or less homogenous. As the result, two hospitals, 3 health centers, and 1 private clinic were selected at random using the lottery method presuming that there is no information lost with the unselected hospitals, health centers, and clinics. Also, the guidelines for the management of tuberculosis work equally for hospitals, health centers, and at the clinic level. So in total, a sampling frame of children managed for TB from 2 hospitals (governmental), 3 health centers (2 governmental and 1 private health center), and 1 private clinic in the town were prepared. There were 431 patients enrolled in anti-TB treatment between the year January 2013 and December 2018. Among those patients, 41 cases did not meet the stated eligibility or inclusion criteria. Of these, 23 patients had incomplete data on treatment outcomes and 18 cases were transferred out to other health facilities. Samples were allotted to each health institution using the probability proportional to size sampling. All childhood TB patients who were registered in selected health facilities and had complete documentation have had an equal chance to participate in the study. The list (unit TB number) of eligible patients on the unit TB register was used as a sampling frame to select the study subjects. The Simple Random Sampling (SRS) technique was used using SPSS software to select 390 samples. Thus, altogether, 390 case files were included in the study (Figure 1).
Figure 1: Schematic presentation of sampling procedure.
Study variables
Poor tuberculosis treatment outcomes in children were the dependent variables whereas socio-demographic factors, pretreatment weight, HIV status, type of TB, HIV Rx status (ART), type of health facilities, patient category, AFB smear result, and nutritional factors were the independent variables.
Data collection tools
The data collection tool was a checklist that was prepared based on routine data registration protocol using the standardized anti-TB treatment entry and follow-up form (Unit TB register logbook) employed by the TB clinic which has been adopted by the Ethiopian federal ministry of health. The structured data extraction format (checklists) was developed from the unit TB register to collect patient information at the selected hospitals, health centers, and clinics. The developed checklists were prepared in English language. The checklists consist of data on demographic variables (age, gender, and address), weight, types of TB, smear result (baseline and follow-up for smear-positive PTB (pulmonary TB) patients), category of TB, treatment year, HIV serostatus and treatment outcomes were extracted from TB unit registers of the hospitals, health centers and clinic.
Data collection procedure
The data were collected by reviewing the registered data on the Unit TB register logbook. It was carried out by reviewing sixyears retrospective data analysis of patients treated under the DOTS program registered and completed their TB treatment from January 2016 and December 2021, in six health facilities of Shashemene town. All forms of TB cases in children registered during the study period in all selected health facilities that were provided DOTS services were included in the study. The Unit TB Registers in all selected health facilities during the period were identified by the principal investigator. Two trained data collectors collected TB patients’ data and then it was entered into a computer program (epi info 7).
Data quality control
Before data collection, a pretest was done in 5% of the sample size population in Dida Boke health center which was not included in the actual sampling. Before the actual data collection period, necessary adjustments were made to the checklists. Two well experienced data collectors (clinical nurses) and one supervisor (Health officer) were recruited. Two days of training were given to the data collectors and the supervisor about the objectives of the study, data collection instruments and procedures, and ethical consideration during data collection. To ensure quality data collection, close supervision was carried out by the principal investigator and the supervisor during the data collection. The completed data abstraction forms were checked for completeness of information by the supervisor and principal investigator on daily bases before leaving the facilities. Furthermore, every day data collectors and supervisors had a meeting with the principal investigator to discuss on documentation and exchange of information.
Data management and analysis
The data were checked for completeness, and consistencies, cleaned, coded, and entered using Epi Info version 7. The data was exported to the Statistical Package for Social Sciences (SPSS) software version 23 for statistical analysis. The TB treatment outcomes were ascertained by reviewing the patient’s data. The outcome of each subject was dichotomized into treatment success or poor.
Descriptive statistics such as median with Interquartile Range (IQR) and mean with Standard Deviations (SD) for continuous variables and frequency (%) for categorical variables were used to investigate the characteristics of the patients.
All factors which have a p-value less than 0.25 in the bivariate logistic regression analysis were included in the final model. Multivariable logistic regression was used to identify independently associated factors of tuberculosis treatment outcomes. A p-value less than 0.05 were considered statistically significant.
Ethical consideration
Approval to conduct this study was obtained from Hawassa university, college of medicine and health science, institutional review board. A letter of support was written from a school of public health to Oromia regional health bureau. A written permission letter was also obtained from Oromia regional state health bureau to the Shashemene town health bureau. Written permission letter was taken from Shashemene town health bureau to study health facilities. Support letter was taken from the medical director of each health facility to TB clinics. The checklists were not containing the name, but only the code of the participants. Confidentiality of information was ensured.
Socio-demographic and clinical characteristics
The majority (93.1%) were treated at the public health DOTS facilities. More than half of the cases were self-referral 232 (59.5%), and screened, diagnosed, and completed their anti-TB treatment with the same health facility, 135 (34.6%) of patients were diagnosed and referred from public health facilities, 15 (3.8%) of patients were from private health facilities and 8 (2.1%) of the patients were suspected and referred by health extension workers to TB clinic.
Out of the total, 201 (51.5%) were female, and the median age of the study participants was 6 (IQR, 3-10) years. The majority of study subjects 143 (36.7%) were within the age group between 0-4 years. 128 (32.8%) of the children treated for TB were in age between 5-9 years, while 119 (30.5%) were aged between 10 and 14 years. The median weight when starting the treatment was 18 kg (IQR, 10-25) and 316 (81%) was urban residents (Table 2).
| Patient characteristics | Number | Percent (%) |
|---|---|---|
| Age category | ||
| 0-4 | 143 | 36.7 |
| 5-9 | 128 | 32.8 |
| 10-14 | 119 | 30.5 |
| Sex | ||
| Male | 189 | 48.5 |
| Female | 201 | 51.5 |
| Residence | ||
| Urban | 316 | 81 |
| Rural | 74 | 19 |
| Source of referral | ||
| HEW | 8 | 2.1 |
| Public HF | 135 | 34.6 |
| Private HF | 15 | 3.8 |
| Self-referral | 232 | 59.5 |
| Category of TB | ||
| New | 373 | 95.6 |
| Lost to follow up | 10 | 2.6 |
| Transfer in | 7 | 1.8 |
| Intensive phase drug type | ||
| RHZE | 352 | 90.3 |
| RHZ | 38 | 9.7 |
| Type of TB | ||
| P/Pos | 37 | 9.5 |
| P/Neg | 213 | 54.6 |
| ETB | 140 | 35.9 |
| Smear result | ||
| PTB+ | 37 | 9.5 |
| PTB- | 155 | 39.7 |
| Smear not done/not registered | 198 | 50.8 |
| HIV Status | ||
| Positive | 27 | 6.9 |
| Negative | 363 | 93.1 |
| Treatment year | ||
| 2013 | 49 | 12.6 |
| 2014 | 44 | 11.3 |
| 2015 | 77 | 19.7 |
| 2016 | 58 | 14.9 |
| 2017 | 83 | 21.3 |
| 2018 | 79 | 20.3 |
PTB: Pulmonary Tuberculosis; EPTB: Extra Pulmonary Tuberculosis
Table 2: Socio-demographic and clinical characteristics of study participants (n=390).
Among registered and analyzed TB patients in children, almost all study participants 373 (95.6%) had not received any anti-TB treatment previously. The rest were categorized as return after default and transfer in cases of 10 (2.6%) and 7 (1.8%) respectively. Regarding to the type of TB, pulmonary smearnegative TB accounted for 213 (54.6%) of childhood TB, EPTB accounted for 140 (35.9%), and pulmonary smear-positive TB accounts for 37 (9.5%) (Table 3). Out of a total of 37 children with smear positive PTB, sputum smear microscopy was done for 31 (83.8%) at the end of the second month of treatment and 5 (13.5%) were documented as smear-positive. At the end of the 5th month, 3 (8.1%) were smear-positive, and also at the end of 6th month, 3 (8.1%) of children were smear-positive.
In intensive phase of treatment, 352 (90.3%) of patients were on RHZE drug type and 38 (9.5%) were on RHZ drug type and 382 (97.9%) were on RH drug type and only 1 (0.3%) was RH, E drug type.
Regarding HIV status in children, 27 (6.9%) of the children were positive and 363 (93.1%) were HIV negative. 22 (81.5%) HIV positive were enrolled in HIV care centers and started ART.
Tuberculosis treatment outcomes in children
Treatment success was calculated as the sum of the cases that were cured and completed their treatment. A total of 25 (6.4%) and 331 (84.9%) patients were cured and treatment completed respectively and gives a total treatment success of 356 (91.3%) (Figure 2).
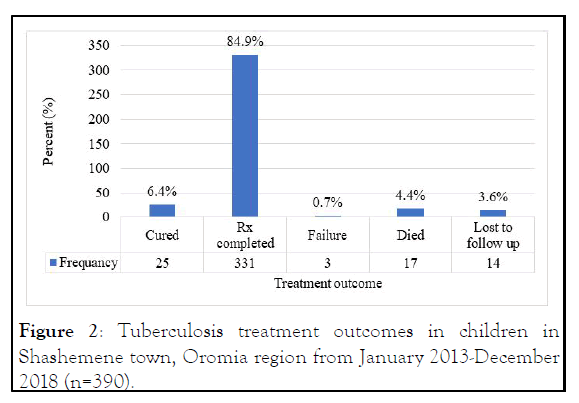
Figure 2: Tuberculosis treatment outcomes in children in Shashemene town, Oromia region from January 2013-December 2018 (n=390).
17 (4.4%) were patients who died, 14 (3.6%) who lost follow-up 3 (0.7%) patients whose treatment failed, and 34 (8.7%) patients had a total poor treatment outcome. The proportion of children that had poor treatment outcomes was significantly higher among children aged 0-4 years (55.8%) compared with those aged between 5-9 years (20.6%) (p-value 0.039) and those 10-14 years (23.5%) (P-value 0.042).
From poor treatment outcomes, the proportion of children that died while receiving treatment (13.3%) and lost to follow-up (6.7%) was significantly higher in those 0-4 years old compared with the other age groups. Deaths were more frequent in under 5 years old children as compared with 5-9 years old, 8 (17%) and 5 (3.8%) deaths respectively, and also there is a high number of deaths in smear-negative pulmonary TB 14 (6.6%) than other forms of TB (2.1%) and in patients categorized as relapse (16.7%) than new cases (4%). There was a high proportion of deaths in rural residents than in urban residents, 5 (6.8%) and 12 (3.8%) deaths respectively.
There were 7 (3.7%) male and 7 (3.5%) female patients were defaulted. The failure rate was higher for male (2 (1.1%) than female (1 (0.5%)) patients. Failure was more frequent in older age groups 10-14 (2 (1.6%)) years old in comparison to 5-9 years (0 (0%)). In HIV co-infected TB patients, the treatment success rate was 19 (70.4%). Out of the total, 1 (3.7%) was cured and 18 (66.7%) were treatment completed. Among HIV co-infected TB patients who had poor treatment outcomes, 6 (22.2%) have died, and 2 (7.4%) were lost to follow-up but had no treatment failure. Out of the total of 17 patients died, more proportion of deaths 6 (35.3%) were recorded among HIV-TB co-infected patients. But, in HIV-negative TB patients the treatment success rate was 93.5%.
Trends in treatment success rate
The trends of TB treatment success rate were variable over the study treatment years. Of a total of TB patients enrolled in anti- TB treatment in 6 health facilities in Shashemene town in 2013, 45 (91.8%) were successfully treated. Of a total of TB patients in children treated in 2014, 40 (90.9%) were successfully treated. Treatment Success Rate (TSR) slightly decreased from 90.9% in 2015 to 89.7% in 2016 and increased to 93.7% in 2018. Therefore, the highest TSR was in 2018 and the lowest in 2016 (Figure 3).
Figure 3: Trends of TB treatment success rate in children across treatment years (n=390) in health facilities of Shashemene Town, Oromia region, January 2013- December 2018.
Factors associated with TB treatment outcomes in children
From the bivariate analysis, there was no significant difference in the treatment outcomes between the sexes (p=0.366). However, residence, age, smear result, category of TB, type of TB, intensive phase drug type, HIV status result, and HIV positive with ART status were significantly associated in this bivariate analysis (p<0.25).
Multivariate logistic regression showed that in children in age group 5-9 years, 10-14 years, category of TB, HIV positive serostatus, and rural residence were independently associated with treatment outcomes.
Patients in age group between 5-9 years had 0.362 times less likely to develop poor treatment outcomes as compared to age group 0-5 years (AOR=0.362, 95% CI (0.138-0.950). Patients in the age group between 10-14 years had 0.354 times less likely to develop poor treatment outcomes as compared to age group 0-5 years (AOR=0.354, 95% CI (0.130-0.963). Patients with the category of lost to follow-up had 8 times more likely to develop poor treatment outcomes as compared to the new category of TB (AOR=8.166, 95% CI (1.437-46.410).
Patients with HIV-positive serostatus who had been treated for TB had a 5 times high risk of developing poor treatment outcomes than HIV-negative patients (AOR=5.822, 95% CI (2.009-16.869).
This study also revealed that people living in rural areas had 2 times more likely to develop poor treatment outcomes than urban residents (AOR=2.390, 95% CI (1.002-5.702) (Table 3).
| Variable | Category | Treatment outcome | COR [95% CI] | p-value | AOR [95% CI] | p-value | ||
|---|---|---|---|---|---|---|---|---|
| Success | Poor | |||||||
| Age | 0-4 | 124 | 19 | 1 | ||||
| 05-Sep | 121 | 7 | 0.378 (0.153-0.931) | 0.034* | 0.362 (0.138-0.950) | 0.039** | ||
| Oct-14 | 111 | 8 | 0.470 (0.198-1.117) | 0.087* | 0.354 (0.130-0.963) | 0.042** | ||
| Residence | Urban | 293 | 23 | 1 | ||||
| Rural | 63 | 11 | 2.224 (1.032-4.796) | 0.041* | 2.390 (1.002-5.702) | 0.049** | ||
| Smear result | PTB+ | 32 | 5 | 1 | ||||
| PTB- | 138 | 17 | 0.788 (0.271-2.296) | 0.663 | 0.896 (0.052-15.385) | 0.94 | ||
| Smear not done/not registered | 186 | 12 | 0.413 (0.136-1.251) | 0.118* | 0.486 (0.027-8.626) | 0.623 | ||
| Category of TB | New | 343 | 30 | 1 | ||||
| lost to follow up | 7 | 3 | 4.900 (1.205-19.931) | 0.026* | 8.166 (1.437-46.410) | 0.018** | ||
| Transfer in | 6 | 1 | 1.906 (0.222-16.353) | 0.557 | 1.407(0.127-15.530) | 0.78 | ||
| Type of TB | P/Pos | 32 | 5 | 1 | ||||
| P/Neg | 194 | 19 | 0.627 (0.219-1.798) | 0.385 | 0.486 (0.028-8.561) | 0.622 | ||
| EPTB | 130 | 10 | 0.492 (0.157-1.541) | 0.224* | 0.663 (0.038-11.569) | 0.778 | ||
| Intensive drug type | RHZE | 324 | 28 | 1 | ||||
| RHZ | 32 | 6 | 2.170 (0.836-5.630) | 0.111* | 1.476(0.507-4.300) | 0.476 | ||
| HIV serostatus | Positive | 19 | 8 | 5.457 (2.181-13.658) | <0.001* | 5.822 (2.009-16.869) | 0.039** | |
| Negative | 337 | 26 | 1 | |||||
*P-value<0.25 COR: Crude Odds Ratio; **P-value <0.05 AOR: Adjusted Odds Ratio PTB+:Smear positive PTB; PTB-:Smear negative PTB; EPTB: Extra pulmonary TB
Table 3: Bivariate and multivariate logistic regression analysis of factors associated with TB treatment outcomes in children in health facilities of Shashemene Town, Oromia region (January 2013-December 2018).
In this study, 390 patients’ records were reviewed. The high proportion of all forms of TB was observed among females (51.5%) than males. This finding was consistent with studies conducted before [19].
Children who were fewer than five constituted the majority of childhood TB in previous studies conducted in Africa and Thailand. Similar to our study, in low-TB-incidence settings, 30%-40% of childhood TB has been reported to occur in children aged less than 5 years [20-22].
In our study, there were a high proportion of pulmonary TB patients (64.1%) which is in line with previous studies; in the Kilimanjaro region, PTB accounted for 75.2%; in Addis Ababa, PTB accounted for 52.6%, and in Abidjan, Cote d'Ivoire, PTB accounted for 74%. This is mainly because most children present with primary rather than secondary TB (with cavitary lesions) and therefore, were likely to have a low bacillary load in sputum. Moreover, young children do not produce sputum for smear microscopy and are diagnosed based on clinical, contact history, and chest x-ray evidence.
The majority of the study participants, 373 (95.6%) were new cases. This shows that lost to follow-up and transferred in cases were small in number and it was in line with studies conducted in Zewditu Memorial Hospital (92.5%), Tigray Regional State (96.5%), in Nigeria (93%).
In this study, the prevalence of TB-HIV co-infection was 27(6.9%), indicating that HIV/TB co-infection was lower than in many previous studies. The less prevalence in our study could be attributed to the improvements in community awareness, implementation of multi-sectoral HIV/AIDS prevention activities, socioeconomic and cultural variability between the study areas.
The overall treatment success of this study was 91.3%, which is in line with the end TB strategy plan of 2025 (90%), WHO Eastern Mediterranean Region treatment success rates (92%), the study done in Kenya (90%) [23]. However, the treatment success rate in our study was higher than the previous studies conducted in Ethiopia and Africa [24]. The treatment success was also higher in our study than in studies conducted before in Mauritania, Nigeria, Australia, and Ethiopia. Our study found that the treatment outcomes of pediatric TB patients treated under the DOTs program were high, in that the treatment success rate is relatively high. The better TB case management, availability of free TB treatment in the study health facilities, and the exclusion of transferred out patients in our study could be the reason for the relatively higher treatment success rate in our study.
The treatment success rate for HIV/TB co-infected children in this study was 70.4% which is consistent with studies in Nigeria 73.4%.
In this study, the poor treatment outcomes of TB in children accounted for 8.7% and the mortality rate was higher (4.4%) in children with poor treatment outcomes. This rate was higher than in the studies have been conducted previously in Ethiopia. The disagreement could be attributed to the difference in sample size and study settings or HIV/TB co-infection and HIV/TB co-infected patients who didn’t on ART.
The treatment success rate of children younger than 5 years was low and showed a significantly greater risk of death from infectious diseases including TB. This could be their immature immune systems. Diagnosis of TB in younger children remains a challenge and most end up with anti-TB treatment without confirmation. This would lead to delay in the diagnosis and treatment of other serious illnesses such as HIV-related opportunistic infections resulting in an increased mortality rate. In addition, the mortality rate in this study was 4.4% which is comparable to the mortality rate reported in Mauritania (4%). However, the rate in our study was lower than in the previous studies. The rate was 5.8% in Malawi, 6% in Nigeria, 5.8% and 5.3% in southern and southern east Ethiopia, respectively and 10.5% in Botswana. These could be due to better TB case management and the availability of free TB treatment in the study health facilities [25].
This study also showed that an overall lost to follow-up rate in the last 6 years was 3.6%. This study is in line with studies done in the Tigray region (3.1%), and Addis Ababa (3.8%). However, this value was higher than in the study has been done at Zewditu memorial hospital (0.6%). Place of residence might be attributed to this relatively high loss of flow up rate.
The majorities of defaulted patients were rural residents. Nevertheless, the loss follow-up rate in our study was lower than studies conducted before in Ethiopia (10.4%) and Mauritania (11%). Patients’ residence, the distance of DOTs sites from their home, socioeconomic status, level of awareness of the disease, and the status of health facilities could be some of the reasons for variation in the findings between these studies.
The treatment failure rate in our study was 0.7%, which is in line with a study conducted at Zewditu memorial hospital (0.4%). It was higher than the study done in Southern Ethiopia (0.2%). The relative higher treatment failure in our study could be due to drug resistance or poor treatment adherence.
Similar to the previous studies, our study also showed an association between HIV negative serostatus and treatment success. HIV co-infection is commonly associated with multiple infections and treatment outcomes that lead to greater morbidity and mortality. The possible reasons for comparatively poor outcomes for HIV positive TB patients in our study could be late detection of HIV-associated TB and MDR-TB, and delays in starting ART or TB treatment. Children are most likely misdiagnosed due to clinical mimicry of other respiratory opportunistic infections such as pyogenic pneumonia and lymphoid interstitial pneumonitis. To reduce excessive TB mortality in subjects who are HIV positive, the WHO recommends routine HIV testing among presumptive and diagnosed TB cases, TB screening among people living with HIV, early ART initiation, improved infection control, and provision of TB preventive treatment. Options that could help to ensure earlier diagnosis and reduce mortality include strategic placement of WHO recommended rapid molecular TB diagnostics such as expert MTB/RIF within HIV care settings.
In this study, pulmonary smear negative TB was more common in pediatric patients than EPTB and pulmonary smear positive TB. It was highly pronounced in the age group below age 5 years.
The overall treatment success rate in this study was high, while HIV/TB co-infection in this study was relatively low. The rate meets the WHO target of 90% and the End TB strategy of 2025. The treatment outcomes were significantly affected by age, HIV status, and patient residence. Poor treatment outcomes were high among children in the age group between 0-4 years, rural residents, and HIV/TB co-infected patients. Most deaths occurred in HIV-positive patients.f registers. The lack of supplementation with qualitative studies was another limitation of our study.
The study was relying on reviewing routinely collected secondary data. Besides, the quality of information obtained from this study was highly dependent on the accuracy and completeness of registers. The lack of supplementation with qualitative studies was another limitation of our study.
Children less than 5 years, HIV/TB co-infected patients, and rural residents need close follow-up during treatment is recommended to all health professionals working at TB clinics Effective collaboration between the TB and HIV programs is needed to implement regular HIV testing for all presumptive and diagnosed TB patients as well as monitoring outcomes of TB-HIV co-infected patients.
We also recommend the expansion of the DOTS service to decrease the patient loss from treatment, especially for rural residents and this accessibility of the DOTS service will further increase the treatment success and reduces the number of lost to follow-up patients. Besides, prospective cohort studies are required to explore various factors affecting TB treatment outcomes in children.
The authors report no conflicts of interest, and all are responsible for the content and writing of this article.
Conceptualization: Habtamu Molla Ayele,
Methodology: Habtamu Molla Ayele, Wondwosen Teklesilasie, Daniel Molla Melese, Abebaye Aragaw
Data collection: Daniel Molla Melese
Formal analysis: Habtamu Molla Ayele,
Supervision: Habtamu Molla Ayele and Wondwosen Teklesilasie
Validation: Wondwosen Teklesilasie, Abebaye Aragaw
Writing original draft: Habtamu Molla Ayele
Writing, review and editing: Habtamu Molla Ayele, Daniel Molla Melese, Wondwosen Teklesilasie Abebaye Aragaw.
Our honest gratitude goes to Oromia region health bureau and Shashemene town health office for the permission to undertake the study and their valuable and relevant information to this research work. Our gratitude also goes to, data collectors, health facility TB focal persons and supervisors for their commitment and hardship resiliency during data collection. We would like to also appreciate the study participants and parents for their voluntary participation in the study.
[Googlescholar][Indexed].
Citation: Ayele HM, Teklesilasie W, Aragaw A, Melese DM (2023) Tuberculosis Treatment Outcomes in Children and Associated Factors in Health Facilities of Shashemene Town, Southern Ethiopia: A Cross-Sectional Study. Clin Pediat. 8:230.
Received: 13-Jul-2022, Manuscript No. CPOA-22-18358; Editor assigned: 15-Jul-2022, Pre QC No. CPOA-22-18358; Reviewed: 29-Jul-2022, QC No. CPOA-22-18358; Revised: 10-Oct-2022, Manuscript No. CPOA-22-18358; Published: 31-Jan-2023 , DOI: 10.35248/2572-0775.22.8.230
Copyright: © 2023 Ayele HM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.