Journal of Leukemia
Open Access
ISSN: 2329-6917
ISSN: 2329-6917
Mini Review - (2023)Volume 11, Issue 5
Therapeutic options for Chronic Lymphocytic Leukemia (CLL) rely on pathway inhibitors targeting Bruton Tyrosine Kinase (BTK) or B Cell Lymphoma 2 (BCL2), while the use of Chemo Immunotherapy (CIT) is limited to contexts where pathway inhibitors are not available or accessible. Biomarkers of treatment refractoriness to CIT include the unmutated status of immunoglobulin heavy chain variable genes and genetic alterations of TP53, NOTCH1, and BIRC3. Acquired mutations of BTK and PLCG2 genes represent the most common resistance mechanisms in patients receiving covalent BTK inhibitors (BTKi)-based therapy. The most frequent BTK mutations are C481S and C481R and impair binding of the drug to its target. Mutations of PLCG2 promote B cell receptor signaling despite BTK inhibition. Recently, several BTK point mutations causing acquired resistance to non-covalent BTKi have been reported, including T474I, L528W, A428D, M437R, and V416L. Concerning BCL2 inhibitors (BCL2i), multiple mutations affecting the BCL2 gene reduce or hinder the binding affinity between the BCL2i venetoclax and the anti-apoptotic BCL2 protein. The G101V substitution is the most common BCL2 mutation and reduces the clinical response to venetoclax. Several additional BCL2 mutations have been detected among CLL patients resistant to venetoclax. Interestingly, acquired 8p loss and 1q gain occur in a fraction of venetoclax-resistant patients and deregulate the expression of the anti-apoptotic MCL1 gene. Leukemic cells characterized by 8p loss and 1q gain display increased resistance to venetoclax and exhibit an augmented sensitivity to MCL1 inhibition. Knowledge of the molecular mechanisms of resistance to pathway inhibitors may help to better understand the sequencing and cross-resistance of available drugs and may contribute to the design of a precision medicine algorithm to be applied to the individual CLL patient.
Chronic lymphocytic leukemia; Treatment refractoriness; Predictive biomarkers; Chemo immunotherapy; Pathway inhibitors
Chronic lymphocytic leukemia, the most common leukemia in the adult population, is defined by the clonal proliferation of mature B cells that express CD19, CD5, and CD23 on their surface and accumulate in the blood and lymphatic tissues [1]. Although CLL displays a 5-year survival rate of approximately 90%, complete eradication of CLL is only achievable with allogeneic hematopoietic stem cell transplantation, a therapeutic approach that is still gravated by a significant rate of morbidity and mortality [2].
For these reasons, therapeutic options for CLL rely on pathway inhibitors targeting Bruton Tyrosine Kinase (BTK) or B Cell Lymphoma 2 (BCL2), while the use of Chemo Immunotherapy (CIT) is limited to contexts where pathway inhibitors are not available or accessible [3]. Currently, available therapies have proven to be effective, but a fraction of patients are refractory to treatment ab initio or acquire secondary resistance during the clinical course [4]. Additionally, in the context of CLL, up to 10% of the cases develop Richter Syndrome (RS), an aggressive lymphoma characterized by histology consistent with diffuse large B cell lymphoma and by frequent refractoriness to CIT [5].
In CLL, several biomarkers of treatment resistance to CIT have been identified and have been recently reviewed in detail by our group [4]. In brief, the main predictors of treatment failure include the unmutated status of Immunoglobulin Heavy Chain Variable (IGHV) genes and genetic alterations of TP53 [4]. Importantly, because of its role as “guardian of the genome”, TP53 exerts a pro-apoptotic function after cellular exposure to DNA-damaging compounds, including CIT. Consequently, TP53 disruption by deletion or mutation increases the resistance of CLL cells to CIT [4]. Genetic alterations of NOTCH1 and BIRC3 also play a role in defining refractoriness to CIT regimens with different mechanisms that, in the case of NOTCH1 mutations, involve reduced levels of CD20 expression and inferior response to some anti-CD20 monoclonal antibodies [4].
To overcome resistance to CIT, BTK inhibitors (BTKi) and BCL2 inhibitors (BCL2i)-based therapies have now become the mainstay of CLL therapy. Remarkably, continuous treatment with the BTKi ibrutinib, acalabrutinib, zanubrutinib have obtained similar results in both IGHV mutated and unmutated CLL, documenting that BTKi have the potential to overcome refractoriness due to IGHV unmutated status. Also, TP53 disrupted CLL treated with continuous treatment with BTKi displayed improved outcomes [4]. Conversely, fixed-duration therapies with BCL2i have shown suboptimal results in TP53-disrupted patients, most likely due to the time limit in exposure to the drug. Because of the clinical impact of TP53 alterations and IGHV unmutated status, the international guidelines from the International Workshop on Chronic Lymphocytic Leukemia (iwCLL) and European Research Initiative on CLL (ERIC), as well as other guidelines, recommend testing these biomarkers at the time of treatment requirement [6,7].
The precise understanding of the mechanisms of resistance to pathway inhibitors in CLL is still under scrutiny, although several concepts have been progressively clarified. On these grounds, the aim of this mini-review is to focus on the molecular mechanisms and clinical implications of refractoriness to CLL pathway inhibitors, in order to provide updates on the latest findings and input for further research on this topic. Mechanisms of refractoriness to both BTK inhibitors (BTKi) and BCL2 inhibitors (BCL2i) will be addressed.
Refractoriness to BTK inhibitors
BTK is a crucial component of the B Cell Receptor (BCR) signaling pathway, which regulates B cell function, proliferation, and survival (Figure 1) [4]. BTKi are a class of drugs that inhibit BTK and thereby have an anti-proliferative and pro-apoptotic effect on CLL cells. BTKi are divided into covalent and non-covalent inhibitors according to the mechanism of action. Covalent inhibitors include ibrutinib, acalabrutinib, and zanubrutinib and bind via a covalent irreversible bond to a cysteine residue at position 481 (Cys481) in the ATP binding pocket abrogating downstream signaling activity. Non-covalent inhibitors (e.g. pirtobrutinib) bind to the ATP-binding domain or a specific H3 pocket of BTK independent of C481 through reversible weak interactions, such as hydrophobic interactions or hydrogen bonding.
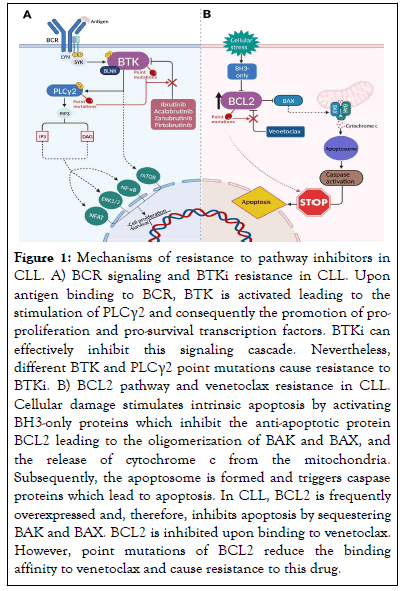
Figure 1: Mechanisms of resistance to pathway inhibitors in CLL. A) BCR signaling and BTKi resistance in CLL. Upon antigen binding to BCR, BTK is activated leading to the stimulation of PLCγ2 and consequently the promotion of proproliferation and pro-survival transcription factors. BTKi can effectively inhibit this signaling cascade. Nevertheless, different BTK and PLCγ2 point mutations cause resistance to BTKi. B) BCL2 pathway and venetoclax resistance in CLL. Cellular damage stimulates intrinsic apoptosis by activating BH3-only proteins which inhibit the anti-apoptotic protein BCL2 leading to the oligomerization of BAK and BAX, and the release of cytochrome c from the mitochondria. Subsequently, the apoptosome is formed and triggers caspase proteins which lead to apoptosis. In CLL, BCL2 is frequently overexpressed and, therefore, inhibits apoptosis by sequestering BAK and BAX. BCL2 is inhibited upon binding to venetoclax. However, point mutations of BCL2 reduce the binding affinity to venetoclax and cause resistance to this drug.
BTKi have been practice-changing in both first-line and Relapsed/Refractory (R/R CLL) [4]. Despite their high efficacy and safety, 60% of patients eventually relapse. Different primary and acquired mechanisms have been described to cause resistance to both covalent and non-covalent BTKi. Baseline characteristics, such as del(17p), TP53 mutation, and complex karyotype, carry a higher risk of progression in ibrutinib-treated CLL [8]. Acquired mechanisms of resistance have been determined during treatment with BTKi or at relapse [9]. In this respect, acquired mutations of the BTK and phospholipase-C-gamma-2 (PLCG2) genes represent the most reported resistance mechanisms in patients receiving covalent BTKi-based therapy (Figure 1). Mutations occur frequently at cysteine residue 481 of BTK, resulting in the replacement of cysteine by other amino acids such as serine (C481S) or arginine (C481R). Mutations at this site lead to the abrogation of covalent binding of ibrutinib, with only transient inhibition of the mutant protein. In contrast, multiple, albeit less frequent, point mutations (i.e. R665W) of the downstream signaling molecule PLCγ2 generally lead to gain-of-function and promote BCR signaling despite BTK inhibition [9].
Using Next Generation Sequencing (NGS), a multicenter international retrospective study by Bonfiglio, et al. showed that only 65% of CLL relapsing on ibrutinib harbor mutations of BTK and/or PLCG2, suggesting the existence of alternative mechanisms of resistance [10]. Interestingly, enrichment of 8p loss has been reported in relapsing cases, leading to haplo insufficiency of TRAIL receptor (TRAIL-R) that causes resistance of leukemic cells to TRAIL-induced apoptosis, independent of the mutational status of BTK and/or PLCG2. Moreover, BIRC3 and NFKBIE mutations were detected exclusively in the BTK wild-type ibrutinib relapsing patients, suggesting aberrant activation of the canonical/non canonical NF-κB pathway as a possible mechanism of drug evasion [10]. Longer follow-up is needed to determine whether the presence of these mutations is associated with subsequent resistance to ibrutinib treatment. Other genetic alterations may complement BTK mutations in inducing BTKi resistance. For example, the transcription factor EGR2 was found to be almost exclusively mutated in relapsed patients carrying BTK mutations [10]. EGR2 is activated by ERK phosphorylation upon BCR stimulation, suggesting that EGR2 mutations may lead to constitutively dysregulated BCR signaling that, together with the existing BTK/PLCG2 mutations, results in ibrutinib resistance.
Non-covalent BTKi are a therapeutic alternative for patients who have failed covalent BTKi [4]. Several mutations causing acquired resistance to non-covalent BTKi have recently been reported, among which point mutations in the tyrosine kinase domain of BTK including T474I, L528W, A428D, M437R, and V416L (Figure 1) [4,11]. A recent study has shown that both gatekeeper and alternative-site mutations in BTK result in resistance to non-covalent BTKi [12]. The BTK gatekeeper mutation T474I has been observed to emerge during pirtobrutinib therapy, replacing the previous C481S clone that had been selected by covalent BTKi. The T474I mutation leads to clinical resistance to non-covalent BTKi. In addition, the T474I clone may also carry an additional BTK mutation (M477I) at a very low frequency which proved to be disabling and was acquired upon progression on pirtobrutinib. In addition to T474I, a previously unreported T474L mutation also resulted in an active BTK [12]. The prevalence of these mutations suggests convergent evolution driven by strong selection pressure, particularly in patients progressing after previous treatment with covalent BTKi. This notion highlights the possible role of reactivation of the proximal component of the BCR pathway as a mechanism of resistance to non-covalent BTKi [11].
An arising problem with covalent and non-covalent BTKi pertains to the emergence of cross-resistance, whereby a single mutation has the potential to confer resistance to both types of inhibitors. This phenomenon significantly complicates the judicious selection of therapeutic interventions. In particular, the BTK L528W mutation demonstrated an increased prevalence in patients who experienced disease progression on treatment with zanubrutinib in comparison to ibrutinib [13]. The same mutation was also detected in several patients progressing during the administration of pirtobrutinib. Interestingly, this mutation also interferes with the binding of adenosine triphosphate, resulting in a protein with reduced kinase activity, rendering it "kinase-dead" but still capable of activating PLCγ2 [13].
Refractoriness to BCL2 inhibitors
BCL2, an anti-apoptotic protein, is overexpressed in CLL allowing cancer cells to evade apoptosis by sequestering pro-apoptotic proteins (Figure 1) [4]. Venetoclax selectively binds to BCL2, displacing pro-apoptotic proteins and triggering apoptosis, and has significantly improved the therapeutic landscape of CLL in combination with anti-CD20 Monoclonal Antibodies (mAbs). Nevertheless, a subset of patients experiences a decline in efficacy and develops secondary resistance to venetoclax [4]. This phenomenon holds true for treatment regimens of both continuous and fixed durations. Specific features of the microenvironment, namely dysregulated expression of IL-10 and CD40L signaling, play a role in venetoclax resistance by triggering Toll-Like Receptor 9 (TLR9) activation, which subsequently initiates NF-κB signaling [14]. It is noteworthy that stimulation of the NF-κB transcription factor is of central importance because it leads to increased levels of the anti-apoptotic proteins BCL-xL and MCL-1 [14]. Baseline genetic lesions also affect venetoclax outcome. Among high-risk CLL patients with TP53 abnormalities, ~50% relapses after 2 years on venetoclax as monotherapy [15]. Also, when venetoclax is combined with the anti-CD20 mAb obinutuzumab, TP53 disruption predicts an inferior outcome most likely due to the fixed duration of the venetoclax-obinutuzumab regimen [16]. In addition, 1q23 amplification, as described by Guièze, et al. can lead to abnormal activation of adenosine monophosphate-activated protein kinase (AMPK)/protein kinase A (PKA), disrupting the release of cytochrome c release and ultimately leading to venetoclax resistance [17].
Multiple mutations affecting the BCL2 gene have been identified as factors contributing to venetoclax resistance by reducing or hindering the binding affinity between the drug and the anti-apoptotic BCL2 protein (Figure 1) [15]. The most common mutation is represented by a substitution of glycine to valine at position G101 (G101V). The presence of BCL2 G101V reduces the cellular response to venetoclax in vitro by altering the BH3-binding domain of BCL2. This mutation is associated with clinical resistance in a maximum of 50% of patients who relapse or progress. In recent years, several additional BCL2 mutations, such as D103Y, which directly disrupts the BH3 binding P4 pocket, have been detected in CLL patients receiving venetoclax [18]. BCL2 D103Y was reported in a recent study to occur also independent of the presence of G101V, which may indicate its role as a driving resistance mechanism to venetoclax [19]. The fact that BCL2 mutations are restricted to a subset of patients suggests the involvement of alternative mechanisms in the emergence of venetoclax resistance [19].
Recently, whole exome sequencing has revealed that certain venetoclax-resistant patients exhibited acquired 8p loss [20]. In tandem, the same patients demonstrated the presence of 1q21.2-21.3 gain which concurrently affected the anti-apoptotic MCL1 gene. Notably, patients characterized by 8p loss displayed heightened resistance to venetoclax in comparison to those lacking such genomic alteration. Intriguingly, cells of patients harboring both 8p loss and 1q21.2-21.3 gain exhibited an augmented sensitivity to MCL1 inhibition, highlighting a possibility for future combinations of MCL1 inhibitors and venetoclax. Furthermore, venetoclax-resistant specimens showed upregulation of the MAPK pERK levels during progression across all patient samples examined [20]. This fact implicates a potentially significant role of MAPK signaling in the development of acquired venetoclax resistance in CLL, as observed in other hematological malignancies, including multiple myeloma and acute myeloid leukemia.
The therapeutic landscape of CLL has been changing rapidly during the last decade thanks to the advent of pathway inhibitors and may continue to evolve in the near future. The availability of both BTKi and BCL2i for first-line treatment as well subsequent lines of therapy poses the question of the optimal sequencing of these medicines during the clinical course of the patient. In this respect, knowledge of the molecular mechanisms of resistance to pathway inhibitors may help to better understand the cross-resistance of available drugs and may in the future contribute to the design of a precision medicine algorithm to be applied to the individual patient. Emerging resistance to pathway inhibitors prompts the exploration of novel pharmaceutical agents, such as Proteolysis-Targeting Chimeras (PROTACS) including BTK degraders, bispecific mAbs and chimeric antigen receptor (CAR)-T cells, for the management of relapsed and refractory CLL. Also, the link between molecular resistance to pathway inhibitors and the development of RS needs to be clarified in more detail, in order to devise better treatment strategies for RS which is still a major unmet clinical need.
Work by the authors described in this review has been supported by Molecular bases of disease dissemination in lymphoid malignancies to optimize curative therapeutic strategies, (5 × 1000 No. 21198), Associazione Italiana per la Ricerca sul Cancro Foundation Milan, Italy; the AGING Project – Department of Excellence – DIMET, Università del Piemonte Orientale, Novara, Italy; Ricerca Finalizzata 2018 (project RF-2018-12365790), MoH, Rome, Italy; Piano Nazionale di Ripresa e Resilienza (PNRR) Missione 6 – Salute (project PNRR-MAD-2022-12375673); and AIL Novara Onlus, Novara, Italy.
N.M. and S.M. declare no conflicts of interest for this specific work. G.G. declares advisory board and speaker’s bureau honoraria from AbbVie, AstraZeneca, BeiGene, Incyte, Janssen, Lilly and Roche.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Maher N, Mouhssine S, Gaidano G (2023) Treatment Refractoriness to Pathway Inhibitors in Chronic Lymphocytic Leukemia. J Leuk. 11:348.
Received: 21-Aug-2023, Manuscript No. JLU-23-26194; Editor assigned: 24-Aug-2023, Pre QC No. JLU-23-26194 (PQ); Reviewed: 12-Sep-2023, QC No. JLU-23-26194; Revised: 19-Sep-2023, Manuscript No. JLU-23-26194 (R); Published: 26-Sep-2023 , DOI: 10.35248/2329-6917.23.11.348
Copyright: © 2023 Maher N, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.