Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2020)Volume 11, Issue 4
Background: VYC-15L is hyaluronic acid filler with relatively low G’ and cohesivity, well suited for the treatment of
infraorbital skin depressions. The present study was intended to assess the safety and effectiveness of VYC-15L in this
indication in real-world clinical practice.
Methods: This was a retrospective review of data from adult patients receiving bilateral treatment of infraorbital skin
depressions using VYC-15L. The injection pattern followed the ‘ 3-point tear trough reshape ’ proposed in the
standardized MD CodesTM methodology, based on points Tt1 (central infraorbital), Tt2 (lateral infraorbital) and Tt3
(medial infraorbital). Pre-treatment with fractional laser was allowed in patients with skin chromophores.
Results: In total, 119 patients were included (mean age: 44.8 ± 12.1 years; n=92 female [77.3%]). Mean injection
quantities of VYC-15L per side were: 0.22 ± 0.06 mL in Tt1, 0.22 ± 0.06 mL in Tt2, and 0.11 ± 0.03 mL in Tt3.
Touch-up was required 2 weeks after initial treatment in 12 patients (10.1%), using 0.1 mL of VYC-15L per side.
Patients reported high levels of satisfaction with outcomes. Eight adverse events were recorded: injection-site
hyperpigmentation, n=5 (4.2%); late edema, n=3 (2.5%). All were mild and resolved without sequelae. Forty patients
(33.6%) received prior treatment with fractional laser; this had no impact on VYC-15L injection quantities or adverse
event profile, suggesting that combined treatment is safe.
Conclusion: Use of VYC-15L for the treatment of infraorbital skin depressions, based on the MD Codes approach,
was safe and effective. This combination of product and technique could become the gold standard.
Filler; Hyaluronic acid; Infraorbital skin depression; Tear trough; VYC-15L; Vycross
The eyes may be the most scrutinized part of the face, not least because the periorbital area contributes greatly to our understanding of emotion in others [1]. The eyes are also the aesthetic center of the face and play a key role in our estimation of age. Indeed, in a study modeling the relationship between facial features and the first impressions of others, four of the five traits that correlated most strongly with youthful attractiveness were features of the eyes [2].
However, the periorbital area is also one of the first parts of the face to show the effects of aging. For example, skin depressions often occur in the tear trough as a result of volume loss in the inferior orbital area and ptosis of malar fat [3,4]. This typically creates a sunken appearance, manifesting as unsightly dark shadows around the lower eyelid [5]. As a result, the face may look sad or tired. Unsurprisingly, therefore, infraorbital skin depressions are often a key area of concern among patients [6].
Hyaluronic acid (HA) fillers represent an important and increasingly popular treatment modality for improving the appearance of infraorbital skin depressions. One such product is VYC-15L, which is based on VycrossTM technology and incorporates a mixture of high- and low-molecular-weight HAs with enhanced cross-linking [7]. Specifically, VYC-15L contains a relatively low concentration of HA (15 mg/mL), thus giving it low G ’ and cohesivity, and hence easy flow and enhanced moldability. It is therefore well suited to use in the tear troughs. In a prospective, multicenter study, VYC-15L was found to be safe and effective for the treatment of infraorbital skin depressions, with results lasting for up to 12 months [8].
It is important to supplement data from prospective clinical trials which typically recruit highly selected patient cohorts with data from routine practice. The present analysis was undertaken in order to provide such data from a sizeable group of patients, many of whom had received prior treatment with fractional laser. Moreover, all patients were injected with VYC-15L based a standardized methodology known as the MD Codes TM [9]. Developed and pioneered by Dr. Mauricio de Maio, the MD Codes technique has been widely adopted in clinical practice in recent years. Studies showing supportive safety and efficacy data are now beginning to appear in the literature [10], but none has yet focused specifically on the periorbital area.
Study design and participants
This was a retrospective review of data from patients receiving treatment of infraorbital skin depressions using the HA filler, VYC-15L (Juvéderm® Volbella with Lidocaine, Allergan, Dublin, Ireland), injected according to the MD Codes technique at a single center between November 2017 and March 2019. The study was conducted in accordance with the Declaration of Helsinki, and all participants provided written informed consent.
Eligible patients were adult males and females wishing to undergo non-surgical correction of infraorbital skin depressions. Key exclusion criteria were previous treatment of the area with fillers within the past 6 months, allergy to any component of the study product, wound healing problems, or any comorbidity that might affect treatment. Women who were pregnant or breastfeeding were also excluded.
Procedures
All study participants were treated bilaterally in a single treatment plan. In most patients, injection of the tear troughs was not performed in isolation but was instead part of full-face treatment. The MD Codes approach which offers a series of practical injection strategies based on specific subunits of the face was applied throughout [9]. In the infraorbital area, this took the form of a ‘3-point tear trough reshape’. These three injection points are known as Tt1 (central infraorbital), Tt2 (lateral infraorbital) and Tt3 (medial infraorbital) (Figure 1). The MD Codes approach also provides suggestions on product, volume, injection technique and device. In this study, injections were made in a sub-orbicularis oculi plane, using a 27G 13 mm needle or 25G 16 mm cannula and a micro-aliquot technique. In most cases, patients received 0.2 mL, 0.2 mL and 0.1 mL of VYC-15L per side in points Tt1, Tt2 and Tt3, respectively.
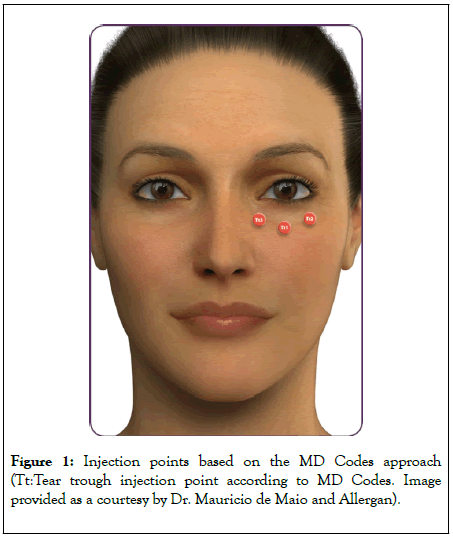
Figure 1: Injection points based on the MD Codes approach (Tt:Tear trough injection point according to MD Codes. Image provided as a courtesy by Dr. Mauricio de Maio and Allergan).
However, if required in the judgment of the physician (e.g. if tear troughs were classed as grade 5 on the Allergan Infra-orbital Scale [AIRS], indicating a defined skin crease from medial to lateral apex, and severe volume loss [11]), patients could receive double quantities, i.e. 0.4 mL, 0.4 mL and 0.2 mL of VYC-15L per side in points Tt1, Tt2 and Tt3, respectively. Touch-ups were made 2 weeks after initial treatment, if necessary, using 0.1 mL per side of VYC-15L.
When injecting point Tt1, care was taken to avoid branches of the infraorbital artery. Similarly, at point Tt3, avoidance of the angular artery and vein was a key safety priority.
A subgroup of patients with skin chromophores were pre-treated with fractional laser using the AcuPulse CO2 laser platform (Lumenis, San Jose, CA, USA). These individuals received four laser sessions, approximately 40 days apart. The final laser session was completed 1 month prior to injection of VYC-15L.
Assessments
Baseline characteristics (age and sex) and VYC-15L treatment volume were recorded for all patients. Results were assessed by the treating physician using standardized pre- and post-treatment photographic images. Adverse events (AEs) were documented during treatment and follow-up visits (scheduled at 2 weeks, 3 months and 6 months post-treatment). Patients were followed for up to 18 months.
Statistical analysis
Descriptive statistics are provided as appropriate, including mean, standard deviation and range for continuous variables, and frequency and percentage for categorical variables.
A total of 119 patients were included in this analysis, with a mean age of 44.8 ± 12.1 years (range: 25-73). Most were female (n=92; 77.3%). Of these 119 individuals, 40 (33.6%) had received prior treatment with fractional laser and 79 (66.4%) had not. Baseline age and sex were similar in the two subgroups (Table 1).
| Characteristic | All patients N=119 | No prior laser N=79 | Prior laser N=40 |
|---|---|---|---|
| Female, n (%) | 92 (77.3) | 60 (75.9) | 32 (80.0) |
| Age, years, mean ± SD (range) | 44.8 ± 12.1 (25–73) | 45.3 ± 12.2 (25–70) | 43.7 ± 12.0 (28–73) |
| VYC-15L injected, mL, mean ± SD (range) | |||
| Tt1 (central infraorbital) | 0.22 ± 0.06 (0.2–0.4) | 0.22 ± 0.06 (0.2–0.4) | 0.23 ± 0.07 (0.2–0.4) |
| Tt2 (lateral infraorbital) | 0.22 ± 0.06 (0.2–0.4) | 0.22 ± 0.06 (0.2–0.4) | 0.23 ± 0.07 (0.2–0.4) |
| Tt3 (medial infraorbital) | 0.11 ± 0.03 (0.1–0.2) | 0.11 ± 0.03 (0.1–0.2) | 0.12 ± 0.04 (0.1–0.2) |
SD: Standard Deviation; Tt: Tear trough Injection Point according to MD Codes.
Table 1: Baseline characteristics and VYC-15L usage.
All patients were treated bilaterally in the tear troughs with VYC-15L, based on the three injection points proposed in the MD Codes. The majority (n=106; 89.1%) received 0.2 mL, 0.2 mL and 0.1 mL of VYC-15L per side in points Tt1, Tt2 and Tt3, respectively; a small number (n=13; 10.9%) instead received 0.4 mL, 0.4 mL and 0.2 mL of VYC-15L per side. Thus, mean quantities were 0.22 ± 0.06 mL, 0.22 ± 0.06 mL, and 0.11 ± 0.03 mL of VYC-15L per side in points Tt1, Tt2 and Tt3, respectively. Quantities used were similar irrespective of whether or not patients had received prior fractional laser therapy (Table 1). Twelve patients (10.1%) received a touch-up 2 weeks after initial treatment, using 0.1 mL of VYC-15L per side, generally in point Tt1.
Example photographs demonstrating the positive impact of treatment with VYC-15L are provided in Figures 2-5. Levels of satisfaction reported by patients were high. With regard to safety, 8 AEs were recorded after treatment with VYC-15L: 5 cases (4.2%) of hyperpigmentation at the injection site, and 3 cases (2.5%) of late edema appearing ≥ 1 month after treatment (Table 2). All were mild and resolved without sequelae. There was no evidence to suggest that AEs were more common in patients with prior laser treatment. Indeed, rates of edema were similar between the two groups (no prior laser: n=2 [2.5%]; prior laser: n=1 [2.5%]), and rates of hyperpigmentation appeared to be slightly lower in those with prior laser versus no prior laser (n=1 [2.5%] vs. n=4 [5.1%,] respectively).
| Event | All patients N=119 | No prior laser N=79 | Prior laser N=40 |
|---|---|---|---|
| Hyperpigmentation | 5 (4.2) | 4 (5.1) | 1 (2.5) |
| Edema | 3 (2.5) | 2 (2.5) | 1 (2.5) |
Data are n (%).
Table 2: Adverse events.
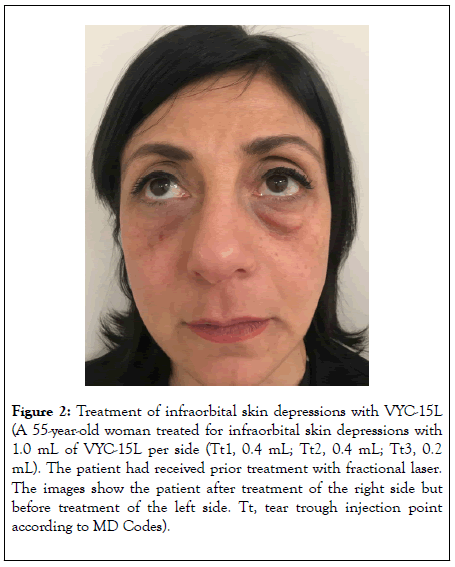
Figure 2: Treatment of infraorbital skin depressions with VYC-15L (A 55-year-old woman treated for infraorbital skin depressions with 1.0 mL of VYC-15L per side (Tt1, 0.4 mL; Tt2, 0.4 mL; Tt3, 0.2 mL). The patient had received prior treatment with fractional laser. The images show the patient after treatment of the right side but before treatment of the left side. Tt, tear trough injection point according to MD Codes).
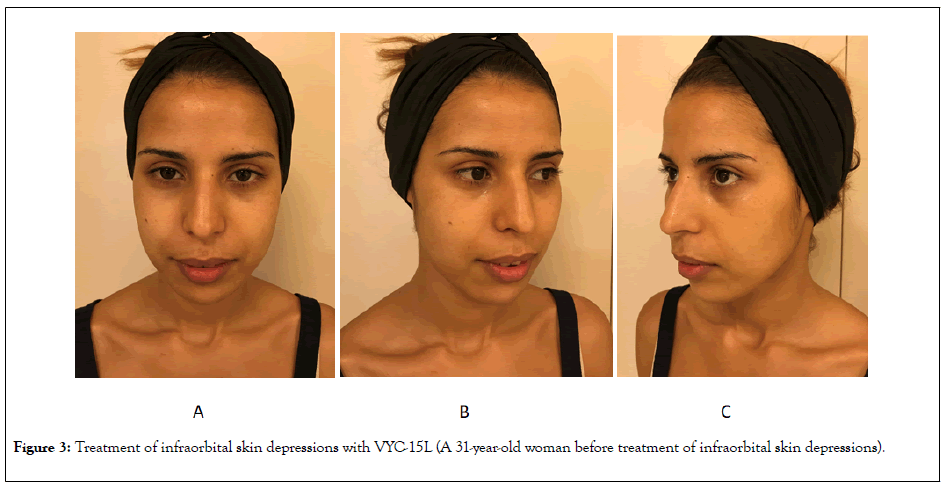
Figure 3: Treatment of infraorbital skin depressions with VYC-15L (A 31-year-old woman before treatment of infraorbital skin depressions).
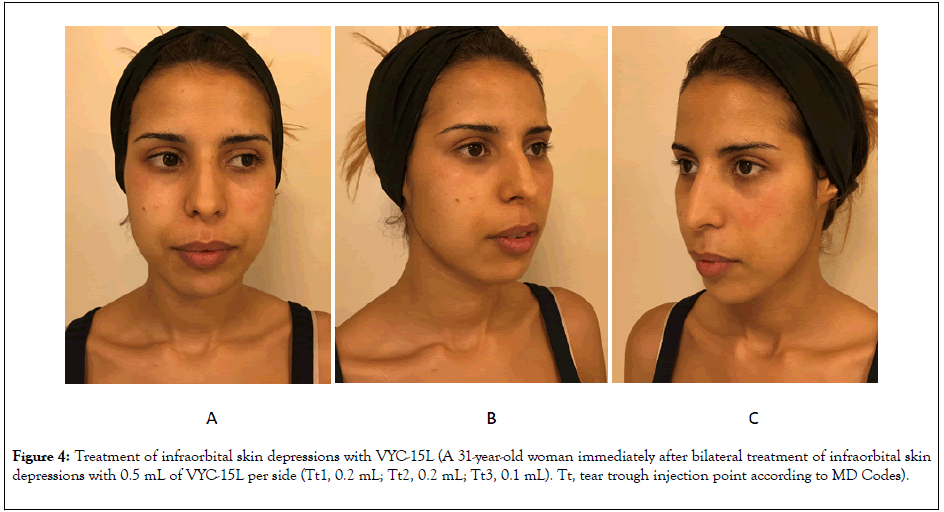
Figure 4: Treatment of infraorbital skin depressions with VYC-15L (A 31-year-old woman immediately after bilateral treatment of infraorbital skin depressions with 0.5 mL of VYC-15L per side (Tt1, 0.2 mL; Tt2, 0.2 mL; Tt3, 0.1 mL). Tt, tear trough injection point according to MD Codes).
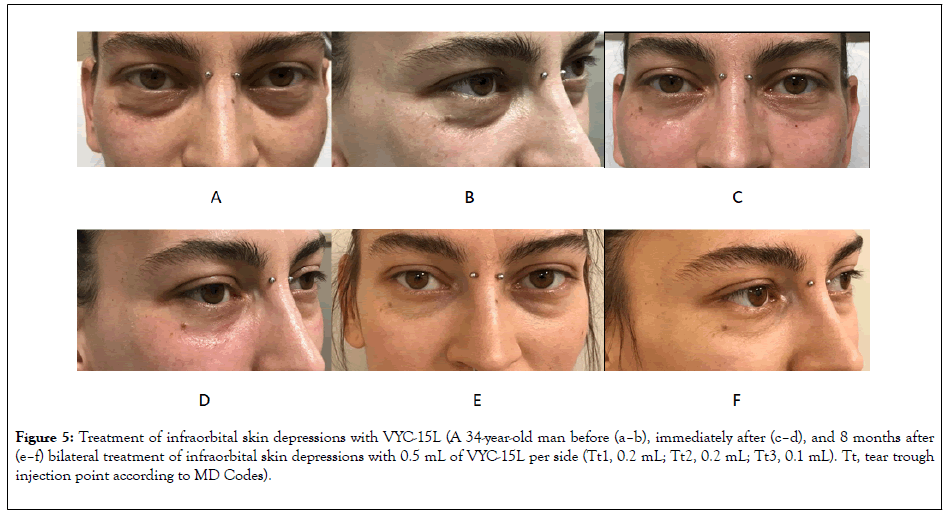
Figure 5: Treatment of infraorbital skin depressions with VYC-15L (A 34-year-old man before (a–b), immediately after (c–d), and 8 months after (e–f) bilateral treatment of infraorbital skin depressions with 0.5 mL of VYC-15L per side (Tt1, 0.2 mL; Tt2, 0.2 mL; Tt3, 0.1 mL). Tt, tear trough injection point according to MD Codes).
To the best of my knowledge, this analysis is the first to assess the safety and efficacy of the MD Codes approach specifically for correcting infraorbital skin depressions. It is also the first assessment of VYC-15L for this indication in a large, ‘real world’ cohort of patients.
The data add to results from a prospective, single-arm study of 80 patients treated with VYC-15L [8]. In that analysis, patients with infraorbital skin depressions rated ≥ 1 on AIRS (i.e. with visible skin creasing and volume loss [11]) received bilateral injections of VYC-15L based on a mean volume of 0.5 mL for initial treatment and 0.3 mL for optional touch-ups at day 14 [8]. At 1 month, improvements of ≥ 1 AIRS grade had been achieved in 99.3% of eyes and response rates remained > 50% out to 12 months. Skin moisture levels were also significantly improved [8].
Safety data from the present analysis also align with those from the prospective trial [8]. AEs were infrequent, localized, mild, and resolved without sequelae. Moreover, the safety profile of VYC-15L is supported by data from multiple prospective trials in other areas of the face, including the lips and perioral area, in which AEs followed the typical profile of transient mild/ moderate edema and bruising [12-17].
Specifically, there were 3 cases of late-onset edema appearing ≥ 1 month after treatment. A higher volume of filler was injected in all of these patients. These data are insufficient to confirm that late edema is related to injection volume, but they do align with the prospective trial – in which injection volume was greater in patients who experienced edema [8]. Various factors should be considered when late edema occurs under the eyes. In particular, patient genetics and electrolyte imbalance (e.g. due to lifestyle or diet) can be important.
There were also 5 cases of hyperpigmentation at the injection site. Here, the pattern was generally unpredictable, although it may be more common in patients with a fair skin phototype (type 1 or 2). Application of depigmenting cream and the use of sunscreen led to complete resolution. However, if hyperpigmentation is still visible after 6 months, laser may be recommended.
In general, the majority of complications with fillers are related to sterility, placement, volume, and injection technique. To reduce complications in the periorbital area, the use of smallvolume, slow, deep injection with massage should be considered to introduce the product gently and evenly [18,19].
The injection pattern and techniques used in this study were based on those proposed by Dr Mauricio de Maio as part of his MD Codes approach [9]. This provides a standardized framework of precisely defined treatment units within each facial area, as well as guidance on the product and volume range, injection device and delivery method, target structures, and key safety points.
Importantly, the MD Codes publication [9] as well as recent consensus recommendations [20,21] – stress that the infraorbital area should only be treated by specialists who are highly experienced and specifically trained in appropriate techniques. They must also have a deep understanding of facial anatomy. Furthermore, appropriate patient selection is crucial, and the importance of a full medical history and details of previous treatments should not be underestimated.
Given the proximity of branches of the infraorbital artery and angular artery and vein, the consequences of injecting this area without adequate knowledge and skill may be particularly grave, including potential necrosis and blindness [19,21,22]. However, as shown in the current series, an appropriately trained and skilled injector can treat this area without encountering serious AEs. The product must be placed below the orbital rim and above the zygomatic region, with deep injections of multiple microboluses (submuscular/preperiosteal). Deep injection may also reduce the risk of edema and Tyndall effect. Nonetheless, filler displacement from deeper to more superficial planes can occur, causing edema [23].
With regard to efficacy in improving infraorbital skin depressions, this was demonstrated in the current cohort by analysis of patient photographs pre- and post-treatment (Figures 2-5). Patients also reported high levels of the satisfaction with aesthetic outcomes. Anecdotally, the effects of treatment appeared to last for 6-9 months. Although data from repeat injections are not presented, many patients did subsequently return for further cycles of treatment of the infraorbital area with VYC-15L. In most cases, the amount of filler required was lower than for initial treatment. This aligns with recent data from a study of repeated use of VYC-15L in the lip and perioral area, in which the volumes of product required were around half those used for initial injection/touch-up [17]. The reasons why reduced volumes of filler are required during re-treatment are not well understood but may relate to stimulation of fibroblasts during initial treatment [24].
The use of fillers is not the only available technique for improving the infraorbital area. Indeed, a number of other methods have been shown to have significant efficacy, including lasers, radiofrequency devices, chemical peels, and topical agents [25]. However, there is relatively little data on the safety and efficacy of combining approaches. In the present study, 40 patients received fractional laser treatment prior to VYC-15L filler injection. Notably, AEs were no more common in these individuals than those who received VYC-15L alone, suggesting that the two modalities can be safely combined to rejuvenate the infraorbital area. The overall aesthetic improvements with combined therapy may be particularly important for patients with dark circles resulting from bilirubin or hemosiderin accumulation.
The limitations of this study should be acknowledged. Most importantly, it was based on data from a single center and had a retrospective design. However, the data accurately reflect the author’s experience and align with results from a prospective, multicenter trial of VYC-15L treatment of infraorbital skin depressions [8]. Neither this analysis nor the prospective trial included a comparator group and hence controlled trials may be a priority for the future.
Overall, the data demonstrate that the use of VYC-15L for the treatment of infraorbital skin depressions, based on the MD Codes approach, is safe and effective. This combination of product and technique may become the gold standard in future.
Writing and editorial assistance was provided by Timothy Ryder, DPhil, of Biological Communications Limited (London, UK) and funded by Allergan plc (now AbbVie) at the request of the investigator. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
Citation: Iera M (2020) Treatment of Infraorbital Skin Depressions using the Hyaluronic Acid Filler VYC-15L based on the MD Codes Approach: A Retrospective Analysis. J Clin Exp Dermatol Res. 11:526. DOI: 10.35248/2155-9554.20.11.526
Received: 26-Jun-2020 Accepted: 10-Jul-2020 Published: 17-Jul-2020 , DOI: 10.35248/2155-9554.20.11.526
Copyright: © 2020 Iera M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.