Hair Therapy & Transplantation
Open Access
ISSN: 2167-0951
ISSN: 2167-0951
Research Article - (2022)Volume 12, Issue 2
Introduction: Androgenetic alopecia also known as male pattern baldness is progressive hair loss characterized by follicular miniaturization in a patterned hair loss occurring due to systemic androgen and genetic factors. AGA has tremendous psychological impact on patients, irrespective of age or the stage of baldness. Androgenetic alopecia in men has been associated with several other medical conditions including coronary heart disease and benign as well as malignant enlargement of prostate, conditions causing insulin resistance such as diabetes, hypertension and obesity.
Materials and methods: The study was conducted in the outpatient department of Dermatology, Venereology and Leprosy in a rural tertiary care hospital of central India for a period of 2 years. 103 clinically diagnosed cases of AGA with age and sex matched controls were subjected to detailed history taking and clinical examination as per prepared questionnaire. In clinical examination BMI, waist circumference and blood pressure was measured and blood investigations included fasting blood glucose, hypertriglyceridemia and HDL cholesterol.
Results: We found a statistically significant difference in prevalence of Metabolic Syndrome (MS) in cases and controls. The prevalence was found to be 21.4% in AGA cases whereas only 1% in controls. We did not find a significant association of severity of AGA with metabolic syndrome. MS was found in 12.62% cases with mildmoderate AGA where as 8.74 % with severe AGA. Risk factors associated with metabolic syndrome like raised BMI was not significantly associated with AGA whereas raised WC was significantly associated with AGA as well as with mild moderate type of AGA. Raised BMI was found in 15.5% of cases whereas WC was raised in 29.1% of cases. AGA was significantly associated with dyslipidaemia. Factors like decreased HDL and raised TG were significantly associated with AGA. HTN (both systolic and diastolic) was not significantly associated with AGA but when we compared cases of AGA with MS both SBP and DBP was found to be significantly raised in mild moderate AGA. Deranged FBS is not associated with AGA.
Conclusion: We conclude that Androgenic Alopecia (AGA) is associated with Metabolic Syndrome (MS). We also conclude that AGA is also associated with individual parameters of MS
Androgenic alopecia; Metabolic syndrome; Hypertension; Fasting blood sugars; Triglycerides
Androgenetic Alopecia (AGA), also known as androgenic alopecia or male pattern baldness, is progressive hair loss characterized by follicular miniaturization in a patterned hair loss occurring due to systemic androgen and genetic factors [1]. The term was first proposed and introduced by Orentreich in 1960 [2]. In the past, the term "androgenetic alopecia" was the primary term used to refer to the appearance of the common, progressive loss of terminal hair on the frontal scalp and/or vertex of the scalp in both men and women. The term "andro" signified a hormonal etiology and "genetic" signified a contribution of heredity to the clinical phenotype [3].
Androgenetic Alopecia (AGA) is a type of pattern alopecia which is an extremely common disorder affecting both sexes but its incidence is generally greater in men than in women. Hair plays a role in psychological communication hence loss of hair has a social impact on an individual’s self-esteem, causing significant psychosocial discomfort [4]. AGA has tremendous psychological impact on patients, irrespective of age or the stage of baldness [5].
Androgenetic alopecia in men has been associated with several other medical conditions including coronary heart disease and benign as well as malignant enlargement of prostate, conditions causing insulin resistance such as diabetes, hypertension and obesity [6].
The study was conducted in the outpatient department of dermatology, venereology and leprosy, in a rural tertiary care hospital of central India for a period of 2 years from November 2018 to October 2020. All consecutive patients with clinical diagnosis of androgenic alopecia presenting in the outpatient of the hospital were enrolled in the study after taking written informed consent. The same number of controls without androgenic alopecia or any other skin conditions were also taken. The source of population for cases and control was taken same. All age and sex matched healthy individuals from the same source of population who were willing to participate in the study were enrolled as controls after written informed consent. After obtaining the informed consent, all patients were subjected to detailed history taking and clinical examination as per prepared questionnaire. In clinical examination height, weight, waist circumference and blood pressure were measured. The Body Mass Index (BMI) was determined by weight and height calculations using the following equation: BMI=weight in kg/square of height in meters (Kg/m2). BMI from 23 to 24.9 is overweight, ≥ 25 is moderate obesity and ≥ 30 is severe obesity. A waist circumference >90 cm for men was considered as abdominal obesity (As per consensus definition of Asian Indians). Blood pressure of >130/85 mmHg was recorded as hypertension. For investigations venous sample was collected from cases as well as control after 8 hours of fasting. Blood samples were evaluated for fasting blood glucose, HDL and triglyceride levels. MS was diagnosed using the South Asian Modified National Cholesterol Education Program Adult Treatment Panel III criteri [7]. If three or more of the following will be present, the cases as well as controls were diagnosed as having MS:
• Abdominal obesity (definition of abdominal obesity was modified using Asia Pacific WHO guidelines as waist circumference ≥ 90 cm for males)
• Blood pressure >130/85 mmHg,
• Fasting blood glucose ≥ 100 mg/dl,
• Hypertriglyceridemia >150 mg/dl, or
• Low HDL cholesterol (<40 mg/dl for males)
Clinical diagnosis of AGA was done using Hamilton Norwood classification (1975). Grading of AGA cases was done as Mild– moderate (grade 1-3) and Severe (grade 4-7) [8]. Similar procedure regarding history, examination and investigations were carried out in age and sex matched controls. Statistical analysis was done by using descriptive and inferential statistics using Chi-square test and student’s unpaired t test and software used in the analysis was SPSS 22.0 version and graph pad prism 7.0. P <0.05 is considered as level of significance. MS was compared between cases and controls and the association between the presence of androgenic alopecia and metabolic syndrome will be tested using odds ratio with 95% confidence interval. P value of less than 0.05 was considered statistically significant.
The present study included 103 cases with clinical diagnosis of AGA and 103 age and sex matched controls. Most common affected age group for AGA was found to be 18-27 years with mean age of 25.78 ± 6.41. We found statistically significant difference in prevalence of Metabolic Syndrome (MS) in cases and controls. The prevalence was found to be 21.4% in AGA cases whereas only 1% in controls (Table 1). In this study we divided cases in mild - moderate (grade 1 to 3) and severe (grade 4-7) with prevalence of 83.5% and 16.5% respectively. MS was found in 12.62% cases with mild-moderate AGA where as 8.74 % with severe AGA (Table 2). We did not find a significant association of severity of AGA with metabolic syndrome. Risk factors associated with metabolic syndrome like raised BMI was not significantly associated with AGA whereas raised WC was significantly associated with AGA as well as with mild moderate type of AGA. Raised BMI was found in 15.5% of cases whereas WC was raised in 29.1% of cases. AGA was significantly associated with factors like decreased HDL and TG. Decreased HDL was found in 10.7% cases, raised TG in 22.3%. HTN (both systolic and diastolic) was not significantly associated with AGA but when we compared cases of AGA with MS both SBP and DBP was found to be significantly raised in mild moderate AGA. Raised SBP was found in 9.7% cases whereas raised DBP was found in 9.7% cases. Deranged FBS is not associated with AGA. Prevalence of deranged FBS was found to be 7.8% (Table 3 and Table 4).
| Metabolic Syndrome | Cases | Controls | |
|---|---|---|---|
| Metabolic Syndrome | 22 (21.4%) | 1 (1%) | 21.58 p=0.0007,S |
| Non Metabolic Syndrome | 81 (78.6%) | 102 (99%) | |
| Total | 103 (100%) | 103 (100%) |
Table 1: Distribution of patients in two groups according to presence of metabolic syndrome.
| Grading of AGA | No of patients | Percentage |
|---|---|---|
| Mild–moderate (grade 1-3) | 86 | 83.5 |
| Severe (grade 4-7) | 17 | 16.5 |
| Total | 103 | 100 |
Table 2: Distribution of cases according to grading of AGA.
The metabolic syndrome is commonly known as a constellation of the obesity, hyperlipidemia, and hyperinsulinemia as well as hypertension. The cluster of these abnormalities is defined as metabolic syndrome, a state associated with increased incidence of several non-communicable diseases (e.g. cardiovascular disease and type 2 diabetes) and all-cause mortality during adulthood. Efforts for early diagnosis of these abnormalities have compelled many clinicians to look for non-invasive clinical markers to diagnose MS. One of it is Frank’s sign i.e. presence of diagonal crease in earlobe. In this study attempt is made to find out association of AGA with MS and its various parameters which can serve as a dermatological clinical marker and various factors which can affect the course of metabolic syndrome in AGA [9]. This is a case control study in which we included 103 cases with clinical diagnosis of AGA and 103 healthy age and sex matched controls.
In the present study, 29.1% cases and 13.6% controls had raised waist circumference. In cases of AGA with MS raised waist circumference was found in 30 cases out of which 22 cases had metabolic syndrome. Out of these 30 cases, 20 (19.42%) belonged to mild-moderate group whereas 10 (9.71%) belonged to severe group (Tables 3 and 4, Figure 1). Difference between the groups was statistically significant (p=0.003, S) giving us clue that raised waist circumference is associated with metabolic syndrome with mild-moderate grades of AGA. Similar findings were obtained by in cases from age group of 20 to 50 years. Findings reported regarding waist circumference parameter are in concordance with our study [10-14].
| Components | Cases (103) | Controls (103) | P value |
|---|---|---|---|
| Fasting blood sugar (≥ 100) | 8 (7.8%) | 1 (1%) | p=0.017, S |
| HDL (<40) | 11 (10.7%) | 3 (2.9%) | p=0.026, S |
| Triglyceride (≥ 150) | 23 (22.3%) | 4 (3.9%) | p=0.0001, S |
| SBP (≥ 130) | 10 (9.7%) | 4 (3.9%) | p=0.09, NS |
| DBP (≥ 85) | 10 (9.7%) | 5 (4.9%) | p=0.18, NS |
| BMI (kg/m2) | 16 (15.5%) | 7 (6.8%) | p=0.0007, S |
| Waist Circumference (>90 cm) | 30 (29.1%) | 14 (13.6%) | p=0.006, S |
Table 3: Distribution of patients in two groups according to presence of components of metabolic syndrome.
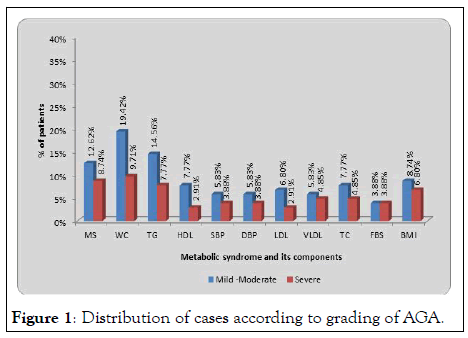
Figure 1: Distribution of cases according to grading of AGA.
In contrast to above findings study done found that no statistically significant association was obtained between waist circumference and metabolic syndrome but he reported statistically significant raised waist circumference in severe AGA as compared to mild AGA. Study reported also reported results contrasting to our study [15].
In the present study, 9.7% cases and 3.9% controls had BP >130 and 9.7% cases and 4.9% controls had BP>85. The difference between the 2 groups in both systolic and diastolic BP is not statistically significant (Table 3)
Whereas out of 10 cases, with raised systolic and diastolic blood pressure maximum no of cases i.e. belonged to mild-moderate group whereas 4 cases belonged to severe group. The difference between the 2 groups is statistically significant (p=0.035, S) this gave us the clue that there is correlation between raised blood pressure and mild-moderate grade of AGA.
Hypertension is found to be associated with AGA and proposed explanation for this association is that there are androgen mediated receptors in arterial wall endothelium. Increase in serum androgen causes smooth muscle proliferation in vessels leading to HTN 15 In our study hypertension was not significantly associated with AGA, this could be due to higher number of cases in younger age group i.e. 18-27 yrs and since majority of the cases belonged to early onset AGA i.e. age below 35 years and majority of our cases were of mild-moderate grade of AGA
Similar findings were observed by whereas contrasting results to our study were found by studies done [16].
Out of total 103 cases and 103 controls, 7.8% cases and 1% control had FBS >100. Whereas among 22 cases of AGA with metabolic syndrome 8 cases had raised FBS levels out of which 4 belonged to mild-moderate group and 5 belonged to severe group. The difference between the groups was not statistically significant (Tables 3 and 4, Graph 4).
Hence it showed that AGA is not associated with FBS. In cases with metabolic syndrome raised FBS was seen in majority in severe cases but it was not statistically significant.
In study done by among 100 cases and controls similar results were obtained. Also reported findings similar to our study. Whereas study done by 15 significant association was reported between AGA and FBS in contrast to our study.
Hyperinsulinaemia with insulin resistance has been reported to be the underlying pathophysiology for metabolic syndrome 17 FBS fails to appropriately reflect it and insulin levels and other parameters of insulin resistance were not studied in our study
In the present study 11 (10.75%) cases and 3 (2.9%) controls have decreased HDL levels and the difference between the two groups was statistically significant (Table 3). Out of these 11 cases maximum i.e. cases belonged to mild to moderate group whereas 3 cases belonged to severe group and the difference was not statistically significant (Table 4, Figure 1). Thus our study showed that AGA is associated with decreased levels of HDL but not associated with severity of AGA. In cross sectional case control study done by show HDL to be significantly decreased in cases of AGA in concordance with our study. Similar findings were observed by [17,18]. Whereas study done by [19] showed no association of decreased HDL with AGA and its severity which is in contrast to our study.
In the present study, 23 (22.3%) cases and 4 (3.9%) controls had TG ≥ 150. Statistically significant difference was found between the 2 groups (Table 3).
Whereas out of 23 cases with raised triglycerides maximum no of cases i.e. 15 belonged to mild-moderate group and 8 cases belonged to severe group and amongst these 23 cases, 22 cases had metabolic syndrome. The difference between the groups was statistically significant (Table 4, Graph 1). This shows that elevated triglyceride levels is associated with mild to moderate grade (1-3) of AGA independent of severity of AGA.
| Metabolic syndrome and its components | Mild -Moderate | Severe | P value |
|---|---|---|---|
| Metabolic syndrome | 13 (12.62%) | 9 (8.74%) | 12.09, p=0.001, S |
| Waist circumference >90 cm (M) or >80 cm (F) | 20 (19.42%) | 10 (9.71%) | 8.69, p=0.003, S |
| Triglyceridemia>150 mg/dl | 15 (14.56%) | 8 (7.77%) | 7.17, p=0.007, S |
| HDL<40 mg/dl (M) or <50 mg/dl(F) | 8 (7.77%) | 3 (2.91%) | 1.03, p=0.30, NS |
| Systolic Blood Pressure | 6 (5.83%) | 4 (3.88%) | 4.43, p=0.035, S |
| Diastolic Blood Pressure | 6 (5.83%) | 4 (3.88%) | 4.43, p=0.035, S |
| Fasting plasma glucose >100 mg/dl | 4 (3.88%) | 4 (3.88%) | 7.06, p=0.008, S |
| BMI(kg/m2) | 9 (8.74%) | 7 (6.80%) | 10.61, p=0.005, S |
Table 4: Distribution of cases according to grading of AGA and metabolic syndrome with its component.
In study done by 10 he reported statistically significant association of TG with AGA similar to our study whereas he also reported raised TG to be statistically significant in severe AGA group which is contrasting to our finding.
In study by 5 TG was not found to be significantly associated with AGA in contrast to finding of our study.
The reason for contrasting results in our study may be due to large no of patients in young age group and with mild-moderate grade of AGA.
On the basis of our study we conclude that Androgenic Alopecia (AGA) is associated with Metabolic Syndrome (MS) and AGA is an independent risk factor for MS irrespective of its severity. We also conclude that AGA is also associated with individual parameters of MS. Early screening and intervention for metabolic syndrome and its parameters in cases of AGA will be beneficial.
[Crossref]
Citation: Patrick S, Kar S, Ramteke K, Nandwani S (2022) To study the Association of Androgenic Alopecia with Metabolic Syndrome and its Individual Parameters in Rural Tertiary Care Hospital in Central Rural India. Hair Ther Transplant. 11:179.
Received: 22-Apr-2022, Manuscript No. HTT-22-17115; Editor assigned: 25-Apr-2022, Pre QC No. HTT-22-17115(PQ); Reviewed: 09-May-2022, QC No. HTT-22-17115; Revised: 22-Jun-2022, Manuscript No. HTT-22-17115(R); Published: 29-Jun-2022 , DOI: DOI: 10.35248/2167-0951.22.11.185
Copyright: © 2022 Patrick S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.