Journal of Sleep Disorders & Therapy
Open Access
ISSN: 2167-0277
ISSN: 2167-0277
Research Article - (2023)Volume 12, Issue 11
Sleep apnoea affects almost one billion people worldwide. Moderate to severe forms of Obstructive Sleep Apnoea (OSA) are associated with an increased risk of cardiovascular and cerebrovascular diseases. Sleep apnoea syndromes are characterized by breathing cessation during sleep. Such hypopnea or apnoeic episodes that last for at least 10 seconds lead to generalized hypoxemia, which not only negatively affects the cardiovascular and neurological systems but also has long-term consequences, including global respiratory failure. Although these episodes do not last for more than 30-50 seconds, it is inevitable that global respiratory failure will develop. Such episodes activate the sympathetic system, creating an undesirable flight-or-fight state, which has negative effects on the sleep cycle. Repetition of these sympathetic states also contributes to nocturnal hypertension, which progresses to essential therapy-resistant hypertension. Consequently, sleep apnoea shortens the estimated average lifespan due to an increased risk of strokes and acute myocardial infarction. Despite the available therapeutic options, including positive pressure ventilation and surgeries, there are no cures or optimal therapeutic options for the prevention of complications. Therefore, the development of new therapies is essential.
The current first-line therapeutic option is a Continuous Positive Airway Pressure (CPAP) machine. Although CPAP has been proven to be a successful treatment for many patients, the issue is patients’ lack of adherence to the therapy. Surgical and conservative treatment options are also available. However, the effectiveness of such therapies has not been proven to be better. A relatively new therapeutic option, Hypoglossal Nerve Stimulation (HNS), is now available. This is a minor surgical intervention in which a pacemaker is inserted to control breathing when apnoea–hypopnea episodes occur. HNS may be the next standard therapeutic option for select patients suffering from OSA. In this article, we systematically reviewed articles addressing OSA, CPAP, and HNS. We discuss the effectiveness of HNS therapy on the Apnoea–Hypopnea Index (AHI), BMI, and systolic blood pressure. We also include the advantages and disadvantages of the therapeutic options currently available for OSA treatment.
Obstructive Sleep Apnoea (OSA); Hypoglossal Nerve Stimulation (HNS); Continuous Positive Airway Pressure (CPAP); Apnoea–Hypopnea Index (AHI); Central Sleep Apnoea (CSA); Obstructive Sleep Apnoea Syndrome (OSAS); Two Diabetes Mellitus (T2DM); Acute Myocardial Infarctions (AMI); Body Mass Index (BMI); Uvulo-Palato-Pharyngoplasty (UPPP).
Sleep apnoea syndromes are characterized by the cessation of breathing during sleep and can include Central Sleep Apnoea (CSA) or Obstructive Sleep Apnoea (OSA) [1]. In CSA, the brain fails to send signals to the breathing system during sleep, leading to a lack of stimulus and breathing cessation. OSA occurs when the stimulus from the brain is initiated during sleep, but respiratory failure occurs due to obstruction of some part of the upper respiratory tract. This article focuses on the latter syndrome. OSA, a heterogenous disease, is a sleep-related breathing disorder characterized by repeated partial or complete obstruction of the upper airways during sleep. This leads to microarousals in the cortex, which create disturbances in normal sleep architecture [2]. According to new guidelines, OSA and Obstructive Sleep Apnoea Syndrome (OSAS) are not interchangeable terms. OSA is a condition in which a patient suffers from hypopnea or apnoeic episodes during sleep but is asymptomatic. On the other hand, OSAS is used to describe symptomatic patients, with symptoms including daytime sleepiness, tiredness, and reduced vigilance [3].
OSA is a major contributor to a reduced quality of life [1]. It can negatively influence work performance due to decreased cognitive function, increase daytime sleepiness, depression, and memory impairment. Furthermore, OSA patients are at an increased risk of motor vehicle accidents, and OSA is associated with increased obesity, hypertension, type Two Diabetes Mellitus (T2DM), coronary artery diseases, strokes, and atrial fibrillations, significantly increasing mortality [1,2].
Epidemiology
The exact epidemiology of people with OSA varies depending on the diagnostic classification method. Previously, an estimated 100 million people were believed to suffer from OSA. However, recent studies have estimated that as many as 936 million patients suffer from OSA—10 times more than the previously assumed prevalence [4,5]. Sleep apnoea affects approximately one billion people worldwide [4,5]. Moderate to severe forms of OSA are associated with a significantly increased risk of cardiovascular and cerebrovascular diseases, showing a positive correlation with Apnoea–Hypopnea Index (AHI) values [6]. The risk of therapy- resistant hypertension, fatal and nonfatal cardiovascular events (e.g., Acute Myocardial Infarctions (AMI) or cardiomyopathies), and all-cause mortality increase significantly in patients with untreated moderate to severe OSA [7,8]. The classification of OSA severity is usually determined by the AHI, which measures the number of apnoea and/or hypopnea episodes throughout the night divided by the number of hours slept. The upper normal limit is five episodes per hour [8].
The prevalence of OSA increased by 30% from 1990 to 2010. Of those affected, 33.9% of men and 17.4% of women aged 30–70 had at least mild OSA (i.e., an AHI score between 5–15 per hour). In addition, 13% of men and 5.6% of women of the same age group suffered from moderate (AHI 15–30 per hour) or severe (AHI >30 per hour) OSA [9]. A fascinating aspect of OSA is the difference in prevalence between genders. Research has consistently confirmed that OSA is more common among men than women [10]. Although the reasons are not well understood, it is believed that inherited differences in fat distribution, length and collapsibility of airways, neurochemical control mechanisms, arousal thresholds, and sex hormones all contribute to this discrepancy [11]. Figure 1 shows the global prevalence of OSA [12].
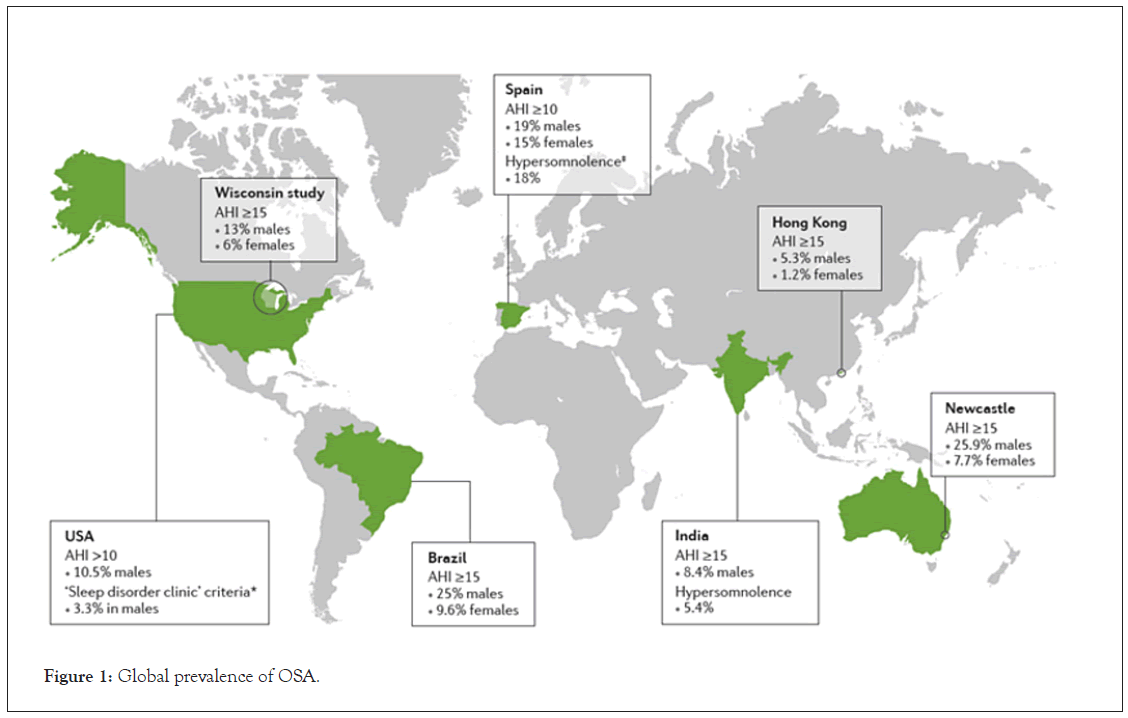
Figure 1: Global prevalence of OSA.
The incidence of developing OSA is strongly associated with Body Mass Index (BMI) and age. However, the association of OSA with BMI and male gender seems to decrease with increasing age, making age the most important factor [1]. Other contributing factors also play a role in an increased risk of OSA development. These include a history of smoking, alcohol consumption, and a positive family history. In addition, endocrinological and chromosomal diseases increase occurrences, including down syndrome, acromegaly, and hypothyroidism [1].Pathophysiology
During physiological sleep, the airways are kept open by the upper airway dilator muscles, most importantly the genioglossal muscle, which is innervated by the hypoglossal nerve. When contracting, these muscles depress the tongue, raise the soft palate, and dilate the oropharynx. The tone and coordination of these muscles are essential in protecting the airways and ensuring intact and sufficient airflow [13]. In OSA, the above-mentioned mechanism fails, causing hypopnea and apnoeic episodes to occur. This is the hallmark of OSA. Regarding the aetiologies of OSA, it is vital to mention the risk factors that increase the chance of developing OSA [14,15]. It has been found that obesity can lead to fat depositions in the pharyngeal fat pads and muscles, which alters the function of the dilatory muscles. Abnormal anatomical craniofacial features due to chromosomal defects such as Down syndrome, birth defects, or traumatic injuries have also been found to increase the risk of OSA [16]. According to previous research, a failure in airway protection during sleep causes intermittent hypoxic episodes. These episodes lead to microarousals in the cortex of the brain, activating the cortex while the patient stays asleep. This phenomenon affects sleep architecture, leading to decreased sleep quality [15].
Apnoeic episodes activate the sympathetic system and interpret it as a flight-or-fight signal due to hypoxia, hypercapnia, and respiratory acidosis. This contributes to a nocturnal hypertensive state, which progresses to therapy-resistant systemic hypertension [15]. In addition, secondary polycythaemia usually manifests in OSA patients due to increased erythropoietin production in response to chronic hypoxemia. Hypercapnia induces an elevation in intracranial pressure, which also aggravates systolic hypertension [17]. The risk of developing pulmonary hypertension also increases significantly. This could be explained by intermittent hypoxemia causing pulmonary vasoconstriction to overcome hypoxemia by increasing blood flow in well-ventilated areas of the lungs [1]. Type Two Diabetes Mellitus (T2DM) has been found to be a major risk factor for OSA. The exact mechanism has yet to be proven. However, it is thought that intermittent hypoxemia during sleep and sympathetic activation promote oxidative stress, which leads to chronic subclinical inflammation. This disrupts the balance between glucose metabolism and insulin secretion. Hypoxemia also plays a role in the pathogenesis of T2DM, as it has a direct effect on the beta cells of the pancreas, promoting apoptosis. Growth hormone and cortisol secretion are also affected by modifying the sensitivity of insulin receptors, leading to insulin resistance. T2DM has its own negative effects on the cardiovascular system: Free radical formation due to oxidative stress can lead to atherosclerosis and its complications, which include coronary artery disease and hypertension [18].Genetic factors and race are additional risk factors for the development of OSA. Genetic factors include craniofacial anatomical variations, obesity, and lung volume. Independent of age, menopause and Body Mass Index (BMI) are also risk factors. The central rearrangement of body fat and the loss of lean muscle mass may link menopause to OSA. Smoking is similarly associated with OSA; it is thought that increased inflammation of the upper airways, nasal congestion, reduced airway sensation, and a reduced arousal threshold could be assumed in the background [9]. The risk factors, their pathomechanisms, and the complications of untreated OSA are summarized and illustrated in Figure 2.
Figure 2: Risk factors, pathomechanisms, and complications of obstructive sleep apnoea (MAP: Mean Arterial Pressure, BP: Blood Pressure, Hr: Heart rate).
Clinical features
Daytime sleepiness, fatigue, and lack of energy are by far the most common symptoms among OSA patients. They are present in up to 90% of patients. Pathological snoring is commonly reported by a third party, such as a spouse or family member. It is also characterized by gasping and choking, which is indicative of OSA; loud snoring is not always present in OSA patients [19]. The severity of daytime sleepiness is measured using the Epworth Sleepiness Scale (ESS), a self-reporting questionnaire consisting of eight questions with a total score of 0–24. A score ≤ 10 is considered normal, while >15 indicates severe daytime sleepiness [19]. Nocturia and mouth dryness have also been reported. The mechanism behind these symptoms involves the release of Atrial Natriuretic Factor (ANF) at night as a response to enhanced negative intrathoracic pressure during gasping and choking, alongside nocturnal hypoxia and hypertension. The body is tricked into perceiving hypervolemia due to an increased sympathetic tone. ANF promotes the excretion of fluids through the kidneys, leading to nocturia. Dryness of the mouth develops due to increased fluid excretion [20]. Morning headaches lasting around 30 minutes are also common. The mechanism behind these headaches is not completely known. Studies suggest that microarousals, hypercapnia, and nocturnal hypertension all play a role [21]. Gastroesophageal reflux due to increased intrathoracic pressure during apnoeic episodes and/or increased intraabdominal pressure towards the chest due to central obesity have also been reported. Decreased libido is another common symptom and can cause depression and social withdrawal. Depression itself has an increased prevalence among OSA patients, while insomnia is common among female OSA patients [22]. Up to 25% of OSA patients do not report daytime sleepiness, categorizing them as asymptomatic. The term ‘asymptomatic’ is often misleading. Patients with OSA may under-report their daytime sleepiness levels due to concerns about professional activities, such as the suspension of their driver’s license [23]. In addition, patients may be desensitized from their symptoms and only realize the negative impact OSA can have on their quality of life after the initiation of treatment [24].
Multiple studies have suggested that, in the USA, untreated hypertension or treated but not sufficiently controlled hypertension lead to a significant increase in the risk of cardiovascular or cerebrovascular mortality. In comparison, treated and well-controlled hypertensive patients have not been found to be at a higher risk of mortality. Symptomless OSA patients with therapy-resistant primary hypertension often find themselves in medical care due to a myocardial infarction or a stroke as a result of undiagnosed hypertension, with no previous warnings [25]. Figure 3 shows survival estimates for all-cause mortality of patients in different groups of hypertensive patients, comparing treated and untreated disease [25].
Figure 3: Kaplan-Meier survival estimates for all-cause mortality
of patients in different groups of hypertensive patients comparing
treated and untreated diseases Note: Hypertension status:  no
hypertension;
no
hypertension;  treated and controlled;
treated and controlled;  untreated;
untreated;  treated but uncontrolled
treated but uncontrolled
Treatment options
Various treatment options aimed at decreasing patients’ symptoms and preventing future complications of untreated OSA exist. However, a definitive cure is not available unless the underlying causes are well established and treatment for those causes is initiated [26].
Many treatment options, both conservative and invasive, are available. These primarily include positive pressure therapy (CPAP, BiPAP), oral appliance therapy, positional therapy, weight loss, behavioural modifications, drug therapy, and upper airway reconstructive surgery. Although these options have documented effectiveness among select patients, treatment effectiveness is frequently incomplete. Thus, new treatment options are needed [27].Recent studies have shown the benefits of turning towards precision medicine [2]. As a guide for clinicians, measuring the complexity of the disease in each patient leads to tailored precision treatments. Such an approach depends on four factors being taken into consideration: disease severity, biological activity, impact on the patient, and the pathophysiological traits of the disease [2]. For patients with mild OSA (AHI <15), conservative treatment is the first-line treatment. This includes weight loss, changes in sleep position, controlling hypertension, and avoiding alcohol, sedatives, or nicotine products, especially before bed, which can exacerbate apnoea or hypopnea episodes. For patients with moderate (AHI <30) to severe (AHI >30) OSA, a different approach, such as surgical intervention and/or ventilatory assistance, is required [8].
Conservative treatment
Continuous Positive Airway Pressure (CPAP) and Bilevel Positive Airway Pressure (BiPAP): The current guidelines suggest Continuous Positive Airway Pressure (CPAP) as the first- line gold standard therapy, as its efficacy is well documented. When comparing CPAP to other modalities, it is by far the most researched modality with proven effectiveness in managing OSA, particularly for multilevel and multifactorial pathophysiological backgrounds. CPAP has been shown to improve snoring, sleep quality, neurocognitive function, daytime sleepiness, and sleep- related life quality [28,29].
This therapeutic method is based on maintaining continuous pressure that constantly stents and protects the airways. The mechanism of action mainly depends on Positive End-Expiratory Pressure (PEEP), which represents the pressure in the alveoli above atmospheric pressure, measured at the end of expiration. CPAP is a way of applying PEEP and of maintaining continuous pressure throughout the respiratory cycle, during both inspiration and expiration. This is measured in centimetres of water pressure (cm H2O) [30].
Despite the known and well-documented efficacy of CPAP in reducing and preventing upper airway obstruction in sleep apnoea, the effectiveness of this therapeutic method is limited and strongly proportional to patient adherence. When ‘adherence’ is defined as greater than four hours of CPAP per night, 46%–83% of patients have been reported to be nonadherent to CPAP, as illustrated in Figure 4 [31,32].
Figure 4: Kaplanâ??Meier curves illustrating different age groups and the
proportion of patients with good continuous positive airway pressure
adherence (at least four hours per night) during follow-up. Note:  65-69 years;
65-69 years;  70-74 years;
70-74 years;  75-94 years;
75-94 years;  = 80
years
= 80
years
Studies documenting the effects of CPAP on sleep apnoea have investigated treatment withdrawal and group differences on multiple indicators of sleep apnoea severity. CPAP withdrawal studies have provided results based on the effect of no treatment (i.e., the amount of time CPAP was not used) on apnoea indicators after a specific period of continuous use. The 2002 dose-response study published by Stepnowsky and colleagues offered an alternative way of assessing the effects of CPAP on sleep apnoea. The focus of the study was adjusted so that the level of compliance the independent variable. Thus, effective CPAP dosing was not only based on an appropriately titrated CPAP pressure but on duration of use and compliance. It was shown that the compliance of patients with CPAP was not as bad as previously reported [33].
There is substantial evidence on the benefits of CPAP for many cardiovascular, pulmonary, and metabolic processes. Such positive benefits likely extend to ‘asymptomatic’ patients if their compliance is adequate. However, there are differences in the magnitude of the responses to CPAP between individuals. According to data from 2019, CPAP therapy should be offered to asymptomatic subjects with moderate to severe OSA as a primary risk prevention [34].
Auto-adjusting Positive Airway Pressure (APAP) machines provide an alternative to CPAP for long-term therapy. Probable advantages include improved comfort and adherence of patients by minimizing unnecessarily high pressure. Earlier smaller studies have suggested that APAP may have some advantages over CPAP in terms of patient adherence. However, more recent studies have shown insignificant differences in adherence between the two [35,36]. For example, a randomized six-week trial including 200 unselected patients demonstrated a difference in adherence of around 0.2 hours per night (12 minutes) and a small difference in daytime sleepiness when comparing APAP and CPAP [37].
Weight reduction: Obesity is a major risk factor for the development and progression of OSA. The prevalence of OSA in obese or morbidly obese patients (BMI >30) is almost twice that of patients with a normal BMI (BMI 18.5 < X ≤ 24.9). Furthermore, patients suffering from mild OSA who gain 10% of their baseline body weight have a six-fold increased risk of OSA. On the other hand, an equivalent weight loss can result in a more than 20% improvement in the severity of OSA [38].
With obesity as a major risk factor, weight loss is understandably beneficial. Weight loss decreases the severity of OSA by decreasing AHI levels [39]. Moreover, after a BMI reduction of more than 5%, a positive effect on inflammatory and metabolic profiles can be detected, and there is a beneficial effect on the systemic blood pressure of patients with moderate to severe OSA, which is comparable to the benefits enjoyed by patients treated with CPAP [40]. In overweight or obese patients with mild to moderate OSA, weight loss is classically targeted by caloric restriction programmes and drugs used for weight control [41].
Comparing CPAP, oral appliances, and weight loss based on symptomatology and AHI value, a small 10-week study of mild to moderate OSA found that CPAP was the most effective. Weight loss was only achieved by half of the conservatively treated patients, revealing the difficulties of the successful implementation of weight loss programmes. Nevertheless, weight loss combined with other therapeutic options has significantly better results [42].
Oral appliances:This therapeutic option is based on a dental device designed to advance the mandible, thereby enlarging and stabilising the oropharyngeal airway. Its effectiveness in treating OSA is lower than that of CPAP [43].
The oral appliances available for OSA treatment are divided into three main categories based on their mode of action. The first includes soft palate lifters, which reduce vibrations from the soft palate. Evidence of their effectiveness is not conclusive [44]. The second category represents Tongue Retaining Devices (TRD), which use suction pressure to hold the tongue in a forward position during sleep, preventing its fall into the pharynx. The third category includes oral appliances that attach the tongue and advance the mandible during the night: Mandibular Advancement Devices (MADs). MADs are the most common oral appliances used for the treatment of OSA [45].
Randomized studies comparing oral appliance therapy and CPAP for the treatment of mild to moderate OSA have suggested that CPAP is the superior treatment [32,46]. However, a recent study of 126 patients with moderate OSA showed that after one month, the two treatment options had similar effects on sleepiness, simulator performance, and disease-specific quality of life, as measured by the Short-Form Health Survey (SF-36). On the other hand, no significant differences in their effectiveness were found when using the Functional Outcomes of Sleep Questionnaire (FOSQ) [47]. Overall, the data indicate that oral appliance therapy can be recommended as a first-line therapy for patients with mild symptomatic OSA with certain obvious etiological causes [48].
Several possible primary contraindications to this therapeutic option exist, including dental insufficiency, periodontal diseases, and active temporomandibular joint disorders [49]. Mucosal dryness, tooth discomfort, hyper-salivation, and increased temporomandibular joint symptoms are common side effects [50].
Drug therapy:The definitive goal of pharmacological therapy is to reduce upper airway muscle tone during sleep, limiting upper airway obstruction. This approach was first proposed in animal models, and a specific potassium channel blocker (AVE0118) was locally administered to the upper airway in pigs to protect the upper airways from collapsing. The efficacy of this agent in human models remains unproven. Pharmacological therapeutic options in this area remain greatly unexplored, but they are strongly recommended to be explored more [51].
Many pharmacological agents for treating OSA have been tested with little success, and the likelihood of effective pharmacotherapy for OSA has been questioned [52,53]. However, intranasal corticosteroid or selective α₁ adrenergic receptor agonist therapy has been reported to decrease AHI. The background for this option is its capability to treat reversable causes of nasal congestion, which is especially relevant in patients with polyps, allergic rhinitis, or nasal mucosal irritation [54,55].
Theophylline has also been found to reduce AHI values in patients with OSA. The mechanism of widening the airways and decreasing the pressure in COPD patients relates to its effectiveness in OSA patients [56].
The effectiveness of progestogens has also been studied. OSA is less common among females; risk increases significantly in post- menopausal women. This is believed to be due to the positive effect of progesterone on ventilatory drive. However, progestogen has not been proven to have a significant effect on OSA [57].
Surgical treatments:
Uvulo-Palato-Pharyngoplasty (UPPP) and palatine tonsillectomy: Uvulo-Palato-Pharyngoplasty (UPPP) was first described by Fujita and colleagues in 1981. Currently, UPPP is the most widely performed surgery for the treatment of OSA in adults [58,59]. Success rates for isolated UPPP surgery as a therapy for OSA are highly variable, influenced by tonsil size and patient BMI [60]. Multiple variations of the technique have been proposed, with similar results. UPPP has been proven to significantly lower CPAP pressure requirements and enhance CPAP compliance [61,62]. The chances of achieving the modern treatment goal of a postoperative AHI of ≤ 5 with UPPP are statically low. This result may be mostly due to selection bias, as patients varied in age, BMI, and severity of OSA [63]. The role of upper airway surgery in general and UPPP in particular as a therapeutic option for OSA remains ambiguous due to limited sample sizes, a lack of agreement on a clear definition of surgical success, and no available blinded studies comparing UPPP and CPAP [64,65]. Moreover, the potential benefits of old-style upper airway surgical procedures must be carefully weighed against potential risk and morbidity due to a lack of high-quality data and the heterogeneity of many surgical procedures [27]. Upper airway surgical treatments for OSAS aim to manipulate and fix dysfunctional pharyngeal anatomy or bypass the pharynx. Options to modify the pharynx diminish the bulk of soft tissue structures that surround and narrow the airways. The goal is to achieve ablation of the pharyngeal soft tissue while indirectly modifying the facial skeleton, from which the soft tissues are suspended [66]. Multiple other surgical therapeutic options are also available, including maxilla-mandibular advancement (MMA), which advances the facial skeleton and pulls the attached soft tissues forward, causing tension that widens the entire pharyngeal inlet [67]. Based on modern research, it is believed that OSA commonly results from a narrowing of the upper airways in multiple different areas (the soft palate, lateral pharyngeal walls, and tongue base) [22]. A combination of procedures used to alter the presence of airway obstruction at different levels may improve the success of surgical interventions [68].
Figure 5 shows the surgical success values between HNS (also known as upper airway stimulation (UAS)) and Expansion Sphincter Pharyngoplasty (ESP), which is similar to UPPP, using the AHI [69].
Figure 5: Comparison of surgical success and AHI values of less than
15, 10, and 5 between the expansion sphincter pharyngoplasty (ESP)
and upper airway stimulation (UAS) cohorts. Significant differences are
denoted by *. Note:  ESP ;
ESP ;  UAS
UAS
Hypoglossal Nerve Stimulation Therapy (HNS): Hypoglossal nerve stimulation therapy is an emerging treatment. In 2014, the US Food and Drug Administration (FDA) approved implantable Hypoglossal Nerve Stimulation (HNS) as a therapeutic option for select patients with moderate to severe OSA. It is commonly chosen as a second-line therapy if adherence to CPAP is low. HNS therapy uses specific unilateral hypoglossal nerve branch stimulation, which causes tongue protrusion and subsequent multilevel upper airway dilation [70]. This electrical stimulation treatment was designed to increase dilator muscle tone and overcome defects in airway neuromuscular control [71].
The idea of HNS has been an ongoing research topic but was only recently recognized as a second-line therapy for OSA [18]. An article from 1997 titled ‘Direct Hypoglossal Nerve Stimulation in Obstructive Sleep Apnoea’ tested the viability of this mechanism in 15 participants. The conclusion from that article states, ‘Direct HG nerve stimulation below the arousal threshold can improve airflow in patients with obstructive sleep apnoea’ [72].
Based on a cohort study, HNS has demonstrated safety and efficacy after 12 months. Patients with moderate to severe OSA who could not tolerate CPAP were chosen for this study [73]. It was proven that if HNS was discontinued, OSA patients did not immediately revert to their baseline. This finding demonstrates that, compared to CPAP, HNS can modify the course of OSA [74].
The HNS system consists of three components: A stimulant cuff electrode circling a distal branch of the right hypoglossal nerve, a pressure-sensing lead positioned within the fourth or fifth right intercostal space to detect the ventilatory effort, and an implantable pulse generator positioned in a subcutaneous pocket below the right mid-clavicle, contralateral to the cardiac pacemaker implantation region (Figure 6). The device detects ventilatory effort and provides synchronized stimulation to the hypoglossal nerve to increase airway muscle tone and dilate the upper airway inlet [75]. The effectiveness and safety of this therapeutic option in CPAP non-adherent patients has been proven to be significant. HNS has a high compliance rate compared to CPAP and significantly improves objective and subjective indicators of sleep quality. No major complications have been recorded [76]. Based on previous reports, it is approximated that after one year of therapy, the compliance of patients using CPAP is between 40%–60%, compared to 86% for those using HNS therapy [77,78]. The criteria for patients who may benefit from HNS have been set according to several cohort studies and experimental series. These criteria include an AHI value of at least 15 and no more than 50, a BMI of ≤ 35, a non-restrictive collapsing type of the soft palate, and CSA not exceeding 25% of the total AHI values [70,73]. Studies have shown no major side effects. Common side effects include pain at the surgical wound and tongue abrasions due to tongue protrusion against the teeth, which can be overcome with painkillers and adaptation. A recurring complication of traditional upper airway stimulation is dysphagia. This is significantly lower with modern HNS device therapy [18,73]. In Table 1, a summary of HNS treatment complications is shown [76]. Several studies published between 2013-2014 used the basis of Polysomnographic (PSG) parameters and quality-of-life measures to validate the effectiveness of HNS therapy in treating OSA. Reductions in both the postoperative AHI and the oxyhaemoglobin desaturation index were detected. In addition, HNS showed improved scores on sleep quality assessment tools, such as the Epworth Sleepiness Scale (ESS) and the functional outcomes of sleep questionnaire. This provides a positive outlook on the effectiveness of HNS therapy [70,73,79]. In a prospective study published by Zhu and colleagues in 2018, the results of two implantation centres in Germany proved that HNS is an effective treatment for patients older than 64 years of age suffering from OSA [80]. Postoperative outcomes were measured using the objective parameters of the AHI and the Overnight Desaturation Index (ODI), which is comparable to previously published results focusing on younger patients. In addition, a significant improvement in excessive daytime sleepiness was noted, and adherence to therapy was still high 12 months post-implantation. No significant differences have been found between the absolute outcome of HNS therapy between older and younger patients, making it a valid therapeutic option, even for elderly patients [80]. The primary limiting issue is the lack of data on long-term effectiveness. Most trials have a one-year follow-up; only the Stimulation Therapy for Apnea Reduction (STAR) trial has follow-ups 48 months post-implantation. All have demonstrated the maintained effectiveness of the HNS therapeutic device and a lack of complications linked with the therapy itself [78,81].
| Side effects | Patients affected (%) | P value |
|---|---|---|
| Pain | 6.20% | P<0.0001 |
| Tongue abrasions | 11.00% | P<0.0001 |
| Internal HNS device malfunction | 3.00% | P<0.0001 |
| External HNS device malfunction | 5.80% | P<0.0001 |
| Other | 7.00% | P<0.0001 |
Table 1: Complications of HNS therapy.
Figure 6: Hypoglossal nerve stimulation therapy.
Figure 7 summarizes the therapeutic options and the treatment protocols that are currently followed for the treatment of OSA..
Figure 7: Potential therapeutic measures and therapeutic steps and protocols for OSA.
Review
Research project:
Clinical background and objectives: The chief aim of our research and meta-analysis was to assess the available evidence with regard to the effects of the modern and upcoming therapeutic method of Hypoglossal Nerve Stimulation (HNS) on the AHI, BMI, and blood pressure values of OSA patients.
Search strategy
We completed our systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [82] and that of the Cochrane handbook [83]. From September 2019 to 18 October 2019, we performed searches in the following databases: Medline, Embase, and CENTRAL with the following key terms: (Sleep apnoea OR OSAS OR SAS) AND (hypoglossal OR nerve stimulation OR HNS OR pacemaker). We did not use any filters. After selection, we manually screened the reference lists of the included studies for other eligible articles.
Selection, eligibility, and data extraction
Following the systematic search process, duplicates were eliminated from the pooled records. Publications were screened in three stages: first by title, then by abstract, and finally by full text. We included studies of adult patients with OSA that applied HNS as a therapeutic measure. They had to report data on the AHI, BMI, and/or blood pressure. Exclusion criteria included studies focusing on children or adolescents, studies without case reports, overlapping populations, animal experiments, reviews, editorials, letters, notes, and conference abstracts without proper data. The following data were extracted: names of first authors, publication year, study design, interventions, duration of study, AHI, BMI, and blood pressure values.
Statistical analysis
For data synthesis, we used the methods recommended by the Cochrane collaboration [83]. Meta-analyses were performed, and the calculated effect sizes were visualized in forest plots. The random effect model was used for meta-analyses with the DerSimonian and Laird weighting method. For our continuous outcomes (BMI, systolic and diastolic blood pressure), we calculated mean pre- and post-treatment differences with 95% confidence intervals (95% CI) to investigate the effects of long- term HNS treatment. Heterogeneity was tested with the χ2 (chi- square) test and the I2 statistics with the Q test. I2 statistics represent the percentage of effect size heterogeneity that cannot be explained by random chance. If the Q test is significant, it implies that heterogeneity among the effect sizes reported in the analysed studies is more diverse than could be explained by random error. The Q test was considered significant if p<0.1. For meta-analyses, comprehensive meta-analysis software was applied. Due to the low number of available studies, Egger’s test for small study effect could not be performed.
Risk of bias and quality of evidence
Risk-of-bias assessment and quality evaluation were first conducted utilizing a simple risk-of-bias assessment tool issued by the national heart, lung, and blood institute (national institutes of health, 2014). We also completed the revised Cochrane’s risk of bias tool for each included study (see Appendix for a summary). This is a complex modern tool that assesses the risk of bias in non-randomized studies (ROBINS-I) [84]. For a general evaluation of the certainty of evidence, we used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system for AHI and BMI values (Table 4) [85,86].
Results of the search and selection process
A total of 6,955 records were identified through a systematic search in Medline, Embase, and CENTRAL. Following the removal of duplicates, we screened 6,172 publications by title and abstract and 65 publications by full text. The flow diagram of the search and selection process is shown in Figure 8, including reasons for exclusion. Nine studies representing 630 patients met the inclusion criteria and provided data for our meta-analyses. Nine studies assessed the efficacy of HNS on AHI and BMI levels and two studies assessed blood pressure [16,69,70,74,87,88,89,90,91,92,93] (Figure 8).
Figure 8: Flowchart illustrating the search and selection processes of the articles included in the research.
The two studies including blood pressure values failed to provide appropriate blood pressure data for meta-analysis. The baseline characteristics of the analysed populations from the nine articles are shown in Table 2.
| Study | Study design | Duration (months) | N | Age (years) | BMI (kg/m2) | AHI (event/hour) | ||
|---|---|---|---|---|---|---|---|---|
| baseline | endpoint | baseline | endpoint | |||||
| Kent, et al. [18] | case-control | 2-8 | 20 | 64.8 ± 12.0 | 26.5 ± 2.4c | 26.8 ± 4.5 | 33.3 ± 13.0 | 5.1 ± 4.3 |
| Huntley, et al. [69] | case-control | 48 | 153 | 59.9 ± 11.46 | 29.4 ± 3.79c | 29.39 ± 2.83 | 36.25 ± 17.42 | 5.8 ± 8.77 |
| Van de Heyning, et al. [70] | case-control | 6 | 31 | 55.1 ± 9.2 | 29.83 ± 2.48c | 30.0 ± 2.7 | 26.1 ± 4.5 | 7.7 ± 4.1 |
| Hofauer B et al. [87] | case-control | 1 | 102 | 56.7 ± 11.3c | 29.4 ± 4.3c | 29.1 ± 3.7 | 32.8 ± 13.9 | 12.6 ± 13.4 |
| Gillespie et al. [88] | case-control | 48 | 126 | 54 ± 10.2c | 28.4 ± 2.6 | 28.6 ± 3.2 | 32.0 ± 11.8 | 30.2 ± 11.0 |
| Rodenstein et al. [89] | case-control | 12 | 10 | 48.0 ± 11 | 29.5 ± 2.0 | 29.6 ± 2.5 | 41.3 ± 13.3 | 15.0 ± 4.8 |
| Sarber et al. [90] | case-control | 3 | 18 | 63 | 31.76 ± 4.18c | 30.43 ± 3.98 | 39.26 ± 4.88 | 10.88 ± 20.52 |
| Steffen et al. [91] | case-control | 12 | 44 | 58.2 ± 9.99 | 28.8 ± 4.07c | 29.3 ± 3.86 | 27.5 | 9,0 |
| Woodso, et al. [92] | case-control | 60 | 126 | 54.5 ± 10.2 | 28.4 ± 2.85c | 28.6 ± 2.5 | 32.0 ± 11.8 | 12.4 ± 16.3 |
Table 2: Baseline characteristics and post-treatment values of the analysed populations chosen for the meta-analysis.
Effects of HNS on AHI in OSA
Our analyses showed that HNS therapy significantly decreases the AHI in OSA (standardized difference-0.472, 95% CI (-0.555; -0.389)). High levels of significance were demonstrated in the fixed and random effect models (p<0.0001). Although the forest plot indicated substantial heterogeneity (I2=97.032%, p<0.001), this new treatment appears to improve the night-time ventilation of OSA patients. High heterogeneity indicates a significant influence of other non-analysed factors, such as age, anatomical characteristics, duration of disease, genetic background, sex, etc. This is summarized in Figure 9.
Figure 9: Forest plot representing the effects of HNS on the AHI in OSA. Note: Squares show the standard difference between the post-treatment and baseline values; the size of the squares reflects the weight assigned to the study. Horizontal bars indicate 95% confidence intervals (95% CI). The blue diamond shows the overall Effect Size (ES) with its corresponding 95% CI.
Effects of HNS on AHI in OSA
Our analyses showed that HNS therapy does not have a positive effect on BMI (standardized difference-0.002, 95% CI (-0.063; 0.058)) between 12–60 months of treatment. Neither the fixed nor the random effect model showed any change in body weight (p=0.948). In addition, our forest plot indicated very low heterogeneity (I2=0.00%, p=0.536). Therefore, this new treatment does not induce weight loss, despite its significant positive influence on the AHI. The very low heterogeneity reinforces these results (Figure 10).
Figure 10: Forest plot representing the effects of HNS on BMI in OSA. Note: Squares show the standard difference between the post-treatment and baseline values; the size of the squares reflects the weight assigned to the study. Horizontal bars indicate 95% confidence intervals (95% CI). The blue diamond shows the overall Effect Size (ES) with its corresponding 95% CI.
Effects of HSN on blood pressure values in OSA patients
The two available studies failed to show an unequivocal effect of HNS therapy on blood pressure in OSA patients within a period of 6–18 months of treatment. Although systolic blood pressure decreased significantly compared to pre-treatment baseline values (mainly in hypertensive patient populations), diastolic pressure failed to change after HNS treatment [74,78,93]. There was not enough data to conduct a meta-analysis (Table 3).
| Study | Study design | Duration (months) | N | Age (years) | BMI (kg/m2) | AHI (event/hour) | Systolic Blood Pressure (mmHg) | Diastolic Blood Pressure (mmHg) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| baseline | endpoint | baseline | endpoint | baseline | endpoint | baseline | endpoint | |||||
| Woodson, et al. [74] | RCT | 18 | 23 | 57.1 ± 10.0 | 28.4 ± 2.4 | 28.1 ± 2.1 | 31.3 ± 12.3 | 9.6 ± 11.3 | 129.1 ± 16.1 | 123.3±12.9 | 80.3± 9.8 | 76.8±10.1 |
| Walia et al. [93] | Prospective study | 6 | 121 | 58.8 ± 11.4 | 28.9 ± 3.4 | ND | 32.6 ± 13.6 | ND | 134.0 (131.4 to 138.0) | 130.5 (127.1 to 133.8) | 81.5 (79.5 to 83.5) | 80.4 (78.4 to 82.5) |
Table 3: Baseline characteristics and post-treatment values of patient populations of studies that reported blood pressure values.
Risk-of-bias assessment of the selected studies and certainty of evidence
With regard to this new treatment, no randomized controlled clinical trials were available. The available studies were uncontrolled before–after studies or pre–post studies. For the risk of bias assessment of such studies, available options include 1) the quality assessment tool of the heart, Lung, and blood institute for non-controlled pre–post studies and 2) Non-Randomized Studies of Interventions (ROBINS-I). According to the former, most of the available studies were ‘fair’, one was ‘poor’, and only a minority of the clinical studies were evaluated as ‘good’. (National Institutes of Health, 2014). ROBINS-I evaluated the available prospective uncontrolled pre–post studies and the retrospective analyses as ‘moderate’ risk [94].
The overall quality of evidence of the analysed data, based on the GRADE approach, is ‘low’, as summarized in Table 4.
| Certainty assessment | No of patients | Effect | Certainty | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | HNS in OSA | SMD (95% CI) | ||
| Apnoea-hypopnea Index (AHI) | ||||||||||
| 9 | Uncontrolled pre–post studies | serious | serious | not serious | not serious | none | 630 | -0.472 (-0.555 to -0.389) | LOW | IMPORTANT |
| Body Mass Index (BMI) | ||||||||||
| 9 | Uncontrolled pre–post studies | serious | not serious | not serious | not serious | none | 630 | -0.002 (-0.063 to 0.058) | LOW | IMPORTANT |
Table 4: GRADE evaluation of articles chosen based on the measured Apnoea-Hypopnea Index (AHI) and Body Mass Index (BMI) values.
Obstructive sleep apnoea is a widespread multi-etiological syndrome affecting approximately one billion people worldwide. It is necessary to devise a therapeutic option that is reliable, patient-friendly, and efficient for a large percentage of these patients. The current first-line therapy for OSA is the CPAP machine. CPAP therapy is highly effective when used properly. However, adherence of at least six hours per night and the ability to cope with the uncomfortable machine are two difficulties OSA patients usually face.
This systematic review and meta-analysis assessed the clinical outcomes of the application of a novel therapeutic tool for OSA: Hypoglossal Nerve Stimulation (HNS). The efficacy of HNS is a significant beneficial decrease in AHI values for OSA patients, indicating that HNS is an effective therapeutic option. On the other hand, HNS appears to have no effect on the body weight (measured by BMI values) of OSA patients if chosen as a mono- therapeutic option. A lack of untreated control groups in the majority of the available studies may have contributed to these results. In the realm of blood pressure analysis, the existing data are insufficient to yield a statistically robust determination regarding the potential effects—whether advantageous or disadvantageous— of HNS on systolic blood pressure. It remains crucial to attain outcomes of unquestionable reliability concerning the complex connection between HNS and blood pressure.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Yamaguchi Mosako, Kato Yamaha (2023). Therapy for Obstructive Sleep Apnea Using Hypoglossal Nerve Stimulation. J Sleep Disord Ther.12:483.
Received: 30-Oct-2023, Manuscript No. JSDT-23-27834; Editor assigned: 01-Nov-2023, Pre QC No. JSDT-23-27834 (PQ); Reviewed: 15-Nov-2023, QC No. JSDT-23-27834; Revised: 22-Nov-2023, Manuscript No. JSDT-23-27834 (R); Published: 01-Dec-2023 , DOI: 10.35248/2167-0277.23.12.483
Copyright: © 2023 Mosako Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.