Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2021)
Background: The Respiratory Syncytial, Influenza and the SARS-CoV-2 viruses have a lot in common and are intertwined as leading causes of respiratory infections (flu) and flu-like infections in humans. The morbidity, mortality and disease burden posed by these diseases across the globe is humongous. The epidemiology, pathophysiology, clinical presentations and sequalae of these respiratory illnesses are also very similar and could be safely described as a spectrum of a disease process ‘syncytial respiratory disease’. There is justification in simultaneously studying, investigating and researching on these diseases given their close similarities and thin differences.
Methods: This is a review of the literature on Case-Management for RSV, Influenza, COVID-19 and the role of Antiviral drugs. A comprehensive search of MEDLINE (from Jan 2019 to January 2021), EMBASE (from Jan 2019 to January 2021), Publics Ovidius Naso (Ovoid), Database of Abstracts of Reviews of Effects and the Cochrane Central Register of Controlled Trials in Issue 1 of 12, January 2021 of the Cochrane Library was performed. Short-listing of titles and abstracts on the basis of their relevance to the review and subsequent data extraction were undertaken independently by two out of the four authors (TSI and PUI). Differences were resolved by mutual consensus.
Results: The Respirtory Syncitial Virus (RSV), Influenza and SARS-CoV2 (COVID-19) virus were all examined in detail. The similarities and differences were x-rayed, including the mechanism of transmission, pathophysiology and pathogenesis of each. The methods of treatment especially susceptibility on antiviral agents were discussed.
Conclusion: The similarity indices between RSV, Influenza and SARS-CoV2 are strong. Non-pharmaceutical and pharmaceutical measures are required for holistic cure and total eradication of these diseases. Effective and efficient antiviral agent(s) are needed for active management of these groups of viruses and science is challenged to this effect.
Influenza; Antiviral drugs; Respiratory syncytial virus; COVID-19
The Respiratory Syncytial, Influenza and the SARS-CoV-2 viruses have a lot in common and are intertwined as leading causes of respiratory infections (flu) and flu-like infections in humans [1- 3]. The morbidity, mortality and disease burden posed by these diseases across the globe is humongous [4,5]. The epidemiology, pathophysiology, clinical presentations and sequalae of these respiratory illnesses are also very similar and could be safely described as a spectrum of a disease process ‘Syncytial respiratory disease’ [6,7]. There is justification in simultaneously studying, investigating and researching on these diseases given their close similarities and thin differences.
SARS-COV-2 virus infection (COVID-19) is relatively new and just over one year since identification, but has caused a colossal loss of human and economic resources across the world [8,9]. According to Johns Hopkins University Global data on COVID-19, the global confirmed cases were 90,944,546 with a total mortality of 1,946,248. New cases across the globe as at 12th January 2021 was 213,905 and evidence showed that there has been a new wave across the globe with more virulent strains resulting in a faster propagation of the disease. The United States of America alone accounts for 25% of the global morbidity and 19% of the deaths across the world [10]. At present, no country of the world is free of COVID-19 pandemic. Scientific machinations and huge resources have been mobilized towards search for mitigation against this pandemic with some measure of success especially in the area of vaccine research. The effort resulted in the availability and approval of some vaccine candidates for global immunization. There are still huge gaps in the effective treatment for COVID-19 especially in the area of case-management which is excusable, given the brief period since disease discovery.
Conversely, Respiratory syncytial virus (RSV) and Influenza viruses have been in existence for over half a century, resulted in three major pandemics and a quasi-one with several deaths and economic losses; yet a definitive management/treatment for the duo remains a mirage [11,12]. The vaccines available for these diseases are not very efficient; hence, Influenza vaccine must be taken annually for the susceptible groups. On the other hand, RSV vaccine has also not been quite efficient. To this end, the management of RSV and Influenza diseases have been largely symptomatic and preventive. In-road into active management of the illnesses with rational anti-viral therapy and other potential treatments has been largely unsuccessful. There is need for an urgent address on the lacuna and therefore the aim of this review is to assess the need for development of anti-viral drugs for the active management of the three diseases RSV, Influenza and COVID-19.
This is a review of the literature on Case-Management for RSV, Influenza, COVID-19 and the role of Antiviral drugs. A comprehensive search of MEDLINE (from January 2019 to January 2021), EMBASE (from January 2019 to January 2021), Publics Ovidius Naso (Ovoid), Database of Abstracts of Reviews of Effects and the Cochrane Central Register of Controlled Trials in Issue 1 of 12, January 2021 of the Cochrane Library was performed. The searches were restricted to studies written in English language. The search strategy used the exploded Medical Subject Headings term “Case-Management for RSV, Influenza and COVID-19”, combined with the second set obtained using the exploded terms “Antiviral drugs”. Reference lists and citation indexes of identified manuscripts were cross-referenced to identify further relevant literatures. Short-listing of titles and abstracts on the basis of their relevance to the review and subsequent data extraction were undertaken independently by two out of the four authors (TSI and PUI). Differences were resolved by mutual consensus.
Respiratory Syncytial Virus (RSV)
Interest in the disease-modifying therapies for RSV has grown but the mainstay of treatment is still supportive, largely self-limiting and palliative with hydration and anti-pyretic in mild diseases, and intensive care and oxygen supplementation for severe diseases. This is in line with the American Academy of Paediatrics (AAP) and National Institute of Health and Care Excellence (NICE) UK guidelines [13,14]. Effective treatment remains elusive for the management of RSV disease 64 years after the first discovery of the disease. It has an estimated world incidence of 33 million in children (the under 5 years) where it ranks first as causative agent for Acute Respiratory Infection (ARI), with mortality of about 200,000 per year [11]. The high morbidity and mortality are recognised among adults and elderly with comorbidities, underlying airway disease and immunocompromised conditions. Palivizumab is the only licensed agent for passive prophylaxis among pre-term infants and high risk patients, no vaccines yet and trials are on-going regarding the efficacy of antiviral drugs in the treatment (Figure 1).
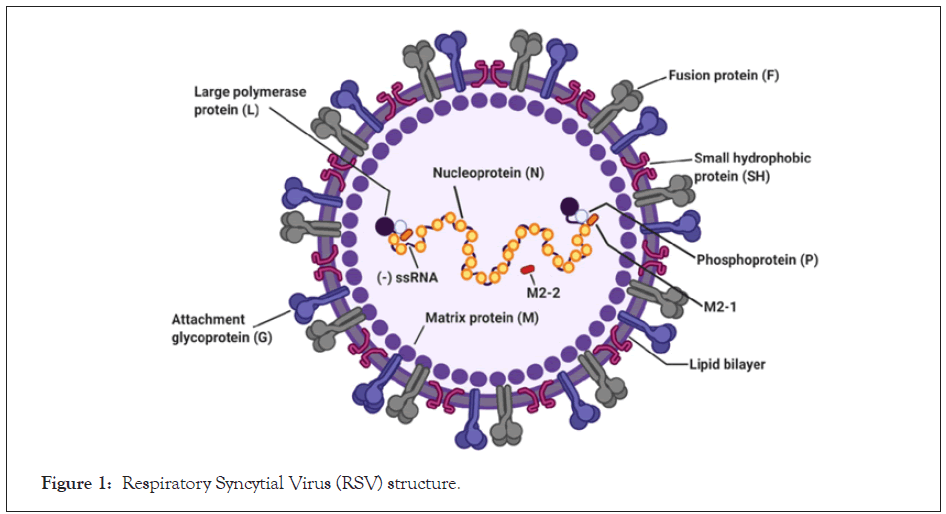
Figure 1: Respiratory Syncytial Virus (RSV) structure.
In developing countries, it ranks second shy of Pneumococcal pneumonia/H. Influenza as cause of lower respiratory tract infection (Pneumonia) in children and this leads to 99% of the global mortality from RSV [11]. Long term sequalae including wheezing, inflammatory airway disease and aggravation of asthma are associated with RSV [15,16]. Real time PCR is an effective molecular level diagnosis for early detection of the disease even with low viral doses. The seasonality (especially during winter in temperate regions and rainy season in the Tropics), pervasiveness among infants/children, adults with underlying respiratory diseases, the elderly, immunocompromised are well established. RSV is an enveloped negative sense, single stranded RNA virus of Pneumovirus family and Orthopneumovirus genus (Figure 1). The virus consists of 11 proteins encoded by a 15.2 kb RSV non-segmented genome and has two genotypes (A and B). It is highly contagious leading to both community and nosocomial infections. The port of entry is the nose or the eyes with predilection for the ciliated upper or lowers respiratory tracts, type 1 pneumocytes and intra epithelial dendritic cells. The incubation period is between 2-8 days. Wide spread and exaggerated inflammation and excessive mucus plugs including sloughing of mucous epithelia, oedema, necrosis, peri bronchial monocyte and T-cell infiltration are pathogenic features of RSV. Syncytial are occasionally formed especially in severe cases and immunocompromised patients [17]. Unlike influenza, RSV does not have the capacity for re-assortment of genome segments and thus to undergo antigenic shifts that have the potential to cause large pandemics [11].
Influenza (The flu)
The world is still searching for the most efficient and effective mode of management for Influenza commonly called ‘The flu’ since 1933, when it was first discovered. Despite passing through three harrowing experiences in the name of pandemics within the 20th century; Spanish influenza in 1918 (17-100 million deaths), Asian influenza in 1957 (two million deaths), and Hong Kong influenza in 1968 (one million deaths) and one quasi pandemic involving H1N1with antecedent human and material losses, finding a definitive solution is still a mirage. The vaccines, treatment regimens are short-termed, since the vaccine protects for approximately one year only [18-21]. The effectiveness of the pills still needs further evaluation and blue-chip research augmentation towards getting the desired outcome.
Influenza is a seasonal disease that affects significant global population with variable degrees of manifestation ranging from mild to severe. CDC estimates that influenza has resulted in 9-45 million illnesses, 140,000-810,000 hospitalizations and 12,000-61,000 deaths annually since 2010 in US alone [22]. All age groups are prone and it is non-gender discriminatory. However, extreme of ages, individuals with underlying illnesses and immunocompromised states are most susceptible. Influenza is caused by Influenza virus, a single stranded RNA entity enclosed in a helical nucleocapsid of Orthomyxoviridae family with four subtypes A-D (Figure 2) [23]. However, the first three namely A, B and C, are the ones with established human infections. The portal of entry is similar to RSV and SARS-CoV-2; being a respiratory disease, and include the nose, eyes and throat. The clinical features and pathogenesis are also similar [24].
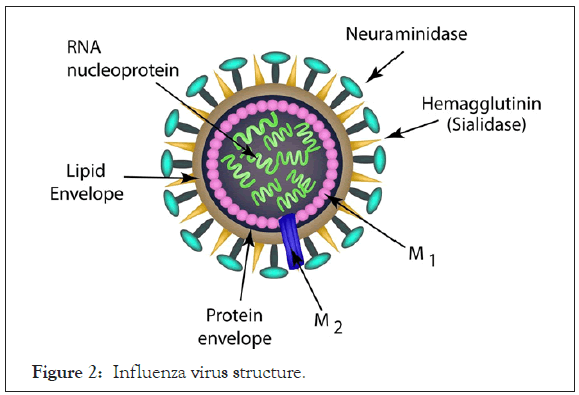
Figure 2: Influenza virus structure.
SARS-CoV2 infection (COVID-19)
This is a relatively new virus, that has barely turned 1year; but its activities and adverse effects witnessed globally, had surpassed several decades of woes [8,9]. It is an enveloped RNA virus, just like RSV and Influenzas virus, except that it is double stranded (Figure 3). Expedited response to this pandemic by the scientific world had resulted in some accelerated discoveries including development of vaccines (mRNA-extracts). The Pfizer, Moderna and Astra Zeneca candidates have all been given an accelerated clearance by the WHO for use, whereas Sputnik V (Russian) and Coax (Indian) candidates are trailing their paths. Despite these giant strides, there are still a lot of conflicts in the pathogenesis and treatment of COVID-19. Lots of controversies in the efficacy of drug candidates including antimalarials, antibiotics and anti-viral drugs. However, one thing is clear, severe COVID-19 disease requires management with some drugs (antivirals etc.) in addition to the supportive care which suffices for mild -moderate cases [25].
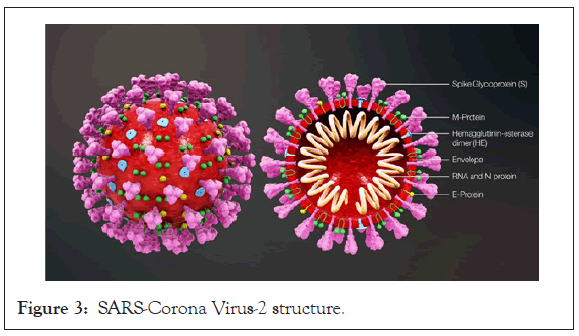
Figure 3: SARS-Corona Virus-2 structure.
The clinical features, mode of transmission, pathogenesis and even sequalae of COVID-19 infection is largely similar to RSV and Influenza disease. Transmission is airborne (aerosol and droplets), contact (with human and contaminated surfaces and enclosures), port of entry via the nose, throat and eyes with affectation of the upper respiratory tracts and complicated with lower respiratory tracts. The spread promoted by crowding and discouraged by physical distancing, use of face masks, hand wash and respiratory hygiene. There are lots of controversies including the theories of conspiracies challenging the origin, propagation and management (including Vaccine) of COVID-19.
Synonyms and points of confluence
The similarities and points of confluence among the three viral diseases-RSV, Influenza and COVID-19; profiled above are very striking [1-3]. These RNA viruses are responsible for major upper and lower respiratory tract diseases in human. They are associated with flu or flu-like diseases of different severities-asymptomatic, mild, moderate and severe disease including complicated cases. The aetiopathogenesis and pathophysiology of these viral diseases are similar and the sequalae converges to the same end point. The management are largely preventative and control through the observation/practices of public health protocols. The segment concerned with active management of the diseases with antiviral drugs is still rudimentary; despite the potential role in the total eradication of the diseases [26]. Active management of the disease by tackling the pathogenic organism is desirable. The passive treatment through vaccine is another important protective arm of management. Given that no vaccination provides 100% protection, it is imperative that the actively infected cases, following vaccine failure or vaccine exclusion, should be treated with relevant therapeutic agents. Therefore, a combination of passive and active treatment modality is very much needed.
Antivirals and related pharmacotherapies
The empirical management of bronchiolitis resulting from RSV has witnessed the use of systemic and inhalational corticosteroids, adrenaline and bronchodilators in adults but not children [27]. Antibiotics for treating super-infections are permitted in children such as Azithromycin, however, the primary agent remains viral. Controversy rages around the use of these steroidal drugs in the immunocompromised.
Currently, Ribavirin is the only licensed antiviral for the treatment of RSV in children with weak evidence [11]. Ribavirin is used in adults especially those with severe illnesses and low immunocompetency including transplant recipients [27]. It tends to be associated with improved outcome, reduced progression to lower respiratory tract infection and decreased overall mortality [28]. Immunoglobulins (RSV specific and non-specific) were also used in the treatment of RSV, but with low level of evidence as elucidated by Cochrane study in 2006 [29]. Montelukast (a leukotriene inhibitor used in treating asthma), was also tried in the management of RSV without palpable significant outcome [30]. Logically, RSV is a viral disease with a well-defined and studied causative agent. Other presentations and features are secondary to this primary infection and therefore, lots of efforts into research should be channelled to find the best antiviral agent that should arrest the activities of this organism in vivo. Further studies on ribavirin, the only antiviral approved for the management of RSV, should aim at modifying and manipulating the structures to be efficacious against RSV.
Influenza virus are known to be susceptible to two classes of antiviral agents namely neuraminidase inhibitors and M2 protein inhibitors. Examples of neuraminidase include Oseltamivir and Peramivir, while amantadine and rimantadine represents M2 protein inhibitors. The pharmaco-kinetics and dynamics of Neuraminidase have been found to be of little benefit towards reducing the duration of the symptoms of the flu. There is no evidence that it prevents or inhibits the propagation to lower respiratory diseases like pneumonia.
The M2 inhibitors are active against viral ion-channel and thus inhibit the replication of the viruses. It is effective against Influenza A but not B, since Influenza B lacks the M2-binding sites [31]. The major problem with M inhibitors lies in resistance formation due to wide use and subtle abuse of the drugs as over the counter drug in the US.
COVID-19 treatment was the most controversial and most experimental. Several candidates including Hydroxychloroquine, Azithromycin, Ivermectin, Lopinavir and Ritonavir were all tried out and appeared to be promising at one time or the other. More rigorous, follow-up researches eventually discredited each of the above drugs and even its combinations.
Up to date, only one antiviral agent has been found worthy and efficacious to gain the approval of the FDA. It is called Remdesivir (GS-5734) and remains the first approved treatment for severe COVID-19. It represents a novel nucleoside analog with broad antiviral activity among RNA viruses including Ebola virus (EBOV) and the respiratory pathogens Middle East respiratory syndrome coronavirus (MERS-CoV), SARS-CoV, and SARS-CoV-2. It was first described in 2016, as derivative from an antiviral library of small molecules targeted on emerging pathogenic RNA viruses. In vivo, Remdesevir showed therapeutic and prophylactic effects in animal models of EBOV, MERS-CoV, SARS-CoV, and SARS-CoV-2 infection [32]. However, in human it was not as successful for this avalanche of RNA viruses. It showed a good result against SAR-CoV-2 and the phase 3 trials showed beneficial clinical effects in patients on supplemental oxygen but not necessarily in critically ill requiring mechanical ventilation [33]. This means, that early administration of the drug once a severe case is identified has merit.
Remdesevir has been demonstrated to reduce the period of recovery by 31%. This means a lot for the patients and also the treatment centres to accommodate more patients. In addition, a lower 14-day mortality rate for patients treated with remdesivir has been demonstrated from the Adaptive COVID-19 Treatment Trial (ACTT) study [34].
The drug inhibits viral entry (via the angiotensin-converting enzyme 2 (ACE2) receptor and transmembrane serine Protease 2 (TMPRSS2), viral membrane fusion and endocytosis, or the activity of the SARS-CoV-2 3-chymotrypsin-like Protease (3CLpro) and the RNA-dependent RNA polymerase. Reports have shown that Remdesivir is useful especially in moderate to severe COVID-19. An initial shot of 200 mg and subsequent daily shots of 100 mg for a minimum of 5 days in individuals 40 Kg and above is adequate but could be extended to 10 days depending on severity and condition of patient. More research is needed in this field to develop other antiviral agents that may be as efficacious but with lesser doses or more effective against the SARS-Cov2 infections.
The similarity indices between RSV, Influenza and SAR-CoV2 are strong. Non-pharmaceutical and pharmaceutical measures are required for holistic cure and total eradication of these diseases. Effective and efficient antiviral agent(s) are needed for active management of these groups of viruses and science is challenged to this effect.
Citation: Ibekwe TS, Kwaghe V, Garba HZ, Ibekwe PU (2021) The Role of Antiviral Drugs in the Case-Management of Respiratory Syncytial Virus, Influenza and COVID-19. J Antivir Antiretrovir. S16:002.
Received: 14-Jan-2021 Accepted: 28-Jan-2021 Published: 04-Feb-2021 , DOI: 10.35248/1948-5964.13.s16.002
Copyright: © 2021 Ibekwe TS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.