Immunome Research
Open Access
ISSN: 1745-7580
ISSN: 1745-7580
Research - (2019)Volume 15, Issue 3
Targeted therapy with small molecule such as TKI (tyrosine kinase inhibitor) against EGFR has achieved high response rate and significant clinical benefits in selected group of lung cancer patients in the past decade. One reliable criterion for patient selection is the presence of certain mutations in the EGFR gene such as mutations in exon-19, 20 or 21 in lung cancer patients. In these patients, it is often observed that TKI therapy could be so effective even against late stage tumors, that patients experience rapid relieve of clinical symptoms and stable control of disease progression for an extended period of time, often lasting for month’s even years.
Tyrosine kinase inhibitor; EGFR mutation; Chemotherapy
It has been assumed that the mechanism behind significant disease control is the total inhibition of the kinase function of the targeted TK molecule by the small inhibitors given daily, so that no tumor replication can take place and inhibited tumor cells die of apoptosis. This mechanism would assume that all tumor cells in a tumor contain the targeted mutation, otherwise how are non-targeted tumor cells controlled? Yet the actual measurement of mutation frequency in almost all cases show a much lower rate of mutated cells, often less than 40%. Then, a question arises: why don ’ t the remaining, non-mutated cells replicate in the presence of the drug? This question has not been satisfactorily addressed despite of its obvious existence in many TKI treated cases of cancer. Here we report our observations and treatment process in one lung cancer patient. The patient had 28% EGFR gene mutation and experienced stable disease control over two years of continuous TKI therapy and a rapid rebound of tumor relapse associated with return of clinical symptoms during a brief drug withdraw of 13 days followed by return of tumor and symptom control upon resumption of TKI therapy. These observations support the explanation that there are two types of tumor cell replication in an established tumor, one self-driven through the function of specific mutation that makes the cell cancerous (EGFR in this case) and the other nonautonomous and dependent on the active replication of the former through induction of inflammation. Control of the entire tumor progression depends on the relationship between these two populations of tumor cells. Targeted therapy is effective only when it is able to control most or all of the replication of the autonomously replicating cells. As new drugresistant mutations only develop during tumor cell replication, the reduction of replication of self-driven replicating tumor cells and thus the dependent non-autonomous replication is a high therapy goal in all case where targeted therapy is possible. Lessons from this case underline the significance of identification of the existence and relationship of these two tumor cell populations in all cancer cases and help to develop better cancer management strategies.
A middle aged man experienced persistent fatigue and coughing with occasional bloody sputum in 2016. Hospital examination revealed a lump of 2.5 cm (largest diameter) in the mid-section of the right lung. Biopsy of the right lung nodule confirmed adenocarcinoma. Further examination with PET-CT showed a primary tumor moderately metabolic active with multiple small nodules in both lungs without clear metabolic activities (Figure 1), metastases in the left supraclavicular lymph nodes (largest one measured 1.6 cm in diameter) and bone metastasis at left rib (Table 1). Test of a panel of common lung cancer tumor markers showed slight increase of CEA and Cyfra21-1. The case was deemed a stage IV disease and was subjected to chemotherapy due to lack of mutations that may fir for targeted therapy. After 6 rounds of chemotherapy with pemetrexed and platinum based on recommendation from NCCN guideline for this cancer, there was minimal response initially and the tumor and tumor markers subsequently rebound with continued progression. The patient also experienced physical deterioration that continued chemotherapy seemed impossible. The tumor was then biopsied again and was tested for mutations that may fit for targeted therapy with TKI. A T790M mutation in the EGFR gene with 28% frequency was identified and the patient was put on osimertinib (80 mg/day). The response was rapid. Within few days, the patient experienced clear improvements in physical state. The sensitive tumor marker all dropped significantly within one month of the TKI treatment. From month 8 till month 17 the patient was in stable condition with no disease progression. PET-CT imaging after one year on osimertinib showed disappearance of the supraclavicular lymph nodes metastases without clear shrinkage of the primary lesion (Table 1).
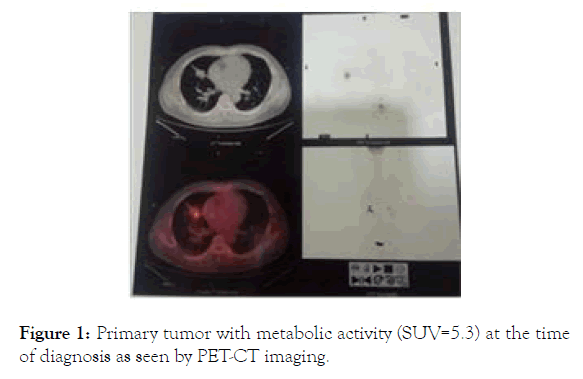
Figure 1. Primary tumor with metabolic activity (SUV=5.3) at the time of diagnosis as seen by PET-CT imaging.
| Month from diagnosis | CEA(0-5) | Cy21-1(0-3.3) | Tumor burden by imaging |
|---|---|---|---|
| 1 | 6.31 | 2.96 | PET-CT: primary tumor at mid right lung, 2.5 × 1.9 cm; multiple nodules in both lungs; right supraclavicular LN, 1.2 × 0.8 cm; left-rear 7th rib metastasis; stage IV |
| 2 | 4.68 | 1.75 | |
| 3 | 6.51 | 3.5 | |
| 4 | 5.61 | 2.43 | |
| 5 | 5.15 | 2.19 | |
| 6 | 5.8 | 3.16 | |
| 7 | 6.46 | 3.33 | CT scan: primary tumor increased to 4.4 × 2.3 cm; multiple nodules in both lungs increased in size; right supraclavicular LN slight increase in size (1.4 × 0.9 cm); rear-left rib metastasis |
| 8 | 2.45 | 2.2 | |
| 17 | 1.52 | 1.78 | |
| 19 | 1.46 | 2.1 | PET-CT: primary tumor of 3.6 × 1.6 cm with low SUV (1.4); multiple small nodules in bith lungs with no metabolic activities; |
| 27 | 2.3 | 2.45 | |
| 35 | 1.68 | 1.53 | |
| 36 | 4.14 | 8.25 | PET-CT: primary tumor 2.5 × 1.9 cm (SUV=5.2);right supraclavicular LN 1.6 × 0.8 cm (SUV=1.7) |
| 36 | 2.91 | 5.17 | |
| 37 | 2.47 | 2.64 | |
| 38 | 3.9 | 4.27 | |
| 39 | 4.9 | 3.67 | |
| 40 | 4.57 | 2.55 | MRI identified possible bone metastasis (persistent bone pain for >3 months) |
| 41 | 2.85 | 1.84 |
Table 1: Treatments and tumor responses.
It was at this time that the patient had come to us seeking advice on how to achieve further response or long-term survival. Since no further response (tumor reduction or eradication) was seen with continued therapy and the side effects of osimertinib was clearly an issue to deal with, it was suggested that maybe the continued daily dosing of osimertinib is not necessary and the nearly complete disease control may also be the action of an antitumor immunity that was activated by TKI therapy initially. This is not impossible since during our clinical study we have seen a number of cases of activation of antitumor immunity by initial TKI therapy (our unpublished results). One way to check for this possibility is to look at the initial biopsy tissue for signs of immune response. However, due to the need for genetic analysis, initial and 2nd biopsy samples were depleted for that purpose thus there was no sample left for us to check this possibility directly. In addition, the long term use of osimertinib and stable but rather dull tumor regression suggest that all target cells were depleted, considering genetic testing showed only a 28% mutation frequency. Then, what were the remaining tumor cells and why didn’t they progress under osimertinib? One clue came from the genetic test on the initial biopsy sample which turned out negative for any of the common lung cancer mutations. That was the reason why the patient was subjected to chemotherapy but not TKI therapy initially. This chain of evidence point to the possibility that the EGFR mutation was not the original reason this tumor developed but developed during progressive growth of the tumor during the initial six rounds of chemotherapy. The possible mechanism of this development will be discussed later in the Discussion section. With this thought, we hypothesized that the long continuous TKI therapy in this case may have already eradicated all of the EGFR mutant cells, leaving the remaining cells not responsive to TKI activity. Since these cells are the original tumor cells dependent on host signal for replication, in the absence of this signal and other stimulus (such as chemotherapy), these cells may remain dormant, thus displaying the long and stable nature of the disease following TKI therapy. To confirm this possibility is critical to the design of subsequent therapy strategy in this case, because a total lack of TKI activity would call for a withdraw for the unnecessary treatment, and a surgery to remove the residual dormant tumor would provide long term prevention of further mutations for self-driven replication. For this reason we suggested withdraw of osimertinib to see whether the above hypothesis is supported. But surprisingly, 3-4 days after withdraw of osimertinib, the patient experienced a gradual increase of discomfort. By one week after withdraw, severe symptoms including tight chest and pain, persistent coughing and low fever had developed that the test of TKI withdraw had to be suspended and therapy was resumed by 13 days after the withdraw. A tumor marker test at the time of TKI therapy resumption showed a clear rebound of all sensitive markers (Table 1) indicating rapid and active tumor proliferation and progression. Few days after resumption of osimertinib, all symptoms subsided gradually and the tumor markers returned to normal range after three weeks on the drug.
This brief relapse of both symptoms and tumor progression following TKI withdraw clearly indicated that despite the fact that the tumor was stable and the patient was clinically uneventful for over 2 years on osimertinib, not all mutant tumor cells that could be targeted by the drug had been eliminated by the treatment. The moment that TKI was stopped, the tumor returned to active self-driven proliferation and typical symptoms associated with lung cancer returned as well. The flare of tumor relapse could be re-controlled by therapy resumption. This brief experience demonstrated the ever-presence of mutant tumor cells capable of self-driven replication but was suppressed by TKI therapy, and rehearses a scenario when drug resistance takes place eventually. In the absence of other potentially effective control of tumor replication, and thus establishment of new metastasis, the only chance that this case may remain on a long disease control would be to reduce the chance of such happening to take place. Significantly reducing existing tumor burden, thus size of replicating tumor cell pool would have direct impact on this chance. In as much as new metastasis is established by single disseminated tumor cell capable of selfdriven replication, such single metastasis rarely has chance to develop any second mutation capable of self-driven replication if the replication of such foci is inhibited effectively by TKI in the first place. The most likely place where drug resistant mutations may develop is in an established tumor nodule where millions of tumor cells are waiting to mutate upon replication under any stimulation. Therefore, reducing visible tumor burden will help to eliminate the base from which any new mutation may develop upon replication. For this, after a PET-CT imaging to confirm lack of active metastases (Figure 2), a surgery to remove residual primary tumor was performed. Post-surgery pathology analyses showed an active tumor. As Figure 3 shows, around 10%-30% of tumor cells were stained positive with Ki-67 (Figure 3, Ki-67).
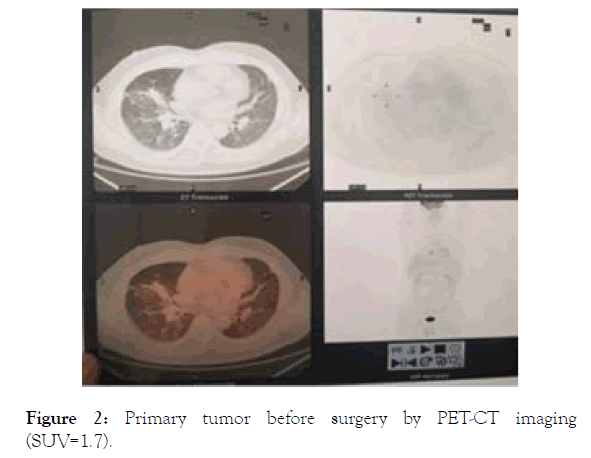
Figure 2. Primary tumor before surgery by PET-CT imaging (SUV=1.7).
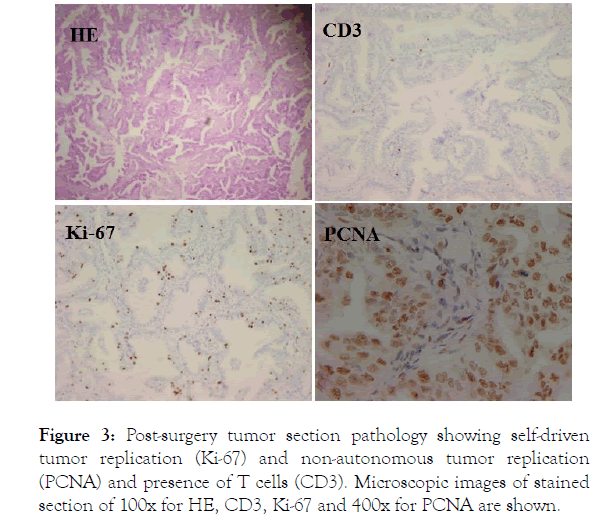
Figure 3. Post-surgery tumor section pathology showing self-driven tumor replication (Ki-67) and non-autonomous tumor replication (PCNA) and presence of T cells (CD3). Microscopic images of stained section of 100x for HE, CD3, Ki-67 and 400x for PCNA are shown.
This is consistent with the previous observation of an active tumor rebound during the brief TKI withdraw. For the preparation of surgery, TKI therapy was stopped a few days before surgery, and this should allow for rapid rebound of tumor replication. The fact that the majority of the tumor cells are replicating is indicated by the positive staining of most tumor cells with another tumor replication marker PCNA (Figure 3, PCNA). Since no more than a third of the tumor cells contain EGFR mutation and are thus capable of self-driven replication, this active replication by a majority of tumor cells in this tumor suggest that most of the replicating tumor cells are not driven by EGFR mutation. When the involvement of antitumor immunity was assessed there was minimal presence of T cells in the resected primary tumor (Figure 3, CD3), indicating lack of antitumor immunity. Because post-surgery recurrence was not protected by antitumor immunity, the patient was put on osimertinib following surgery and remains uneventful since then.
This case has revealed several important aspects in cancer management that deserve further discussion. The first question is how can a mixed tumor cell population with only minority of tumor cells harboring the targeted mutation be controlled completely by drugs that target this mutation specifically? This is rather a representative situation among many patients who are on TKI therapy and experience good response in terms of tumor and symptom control. One has to assume that there must be a dependent relationship between the mutated and the nonmutated portion of the tumor that replication of the latter is dependent on the former. In other words, there are two portions of a tumor in this case, one is self-driven for replication and the other is not. With this assumption, we can explain why in this case osimertinib targeting 28% of the tumor cells was able to obtain moiré than two yearlong complete control of the entire tumor. The next question then is how was this relay in control realized? The tight association of tumor rebound and symptom return suggests that this is through induction of local inflammation. The mutated tumor cells that are capable selfdriven replication concomitantly produce chemotactic factors for inflammation which in turn drives the replication of a large portion of the non-mutated cells. Previous studies have demonstrated this connection between self-driven replication and NFk-B-dependent inflammation and the effect on tumor burden [1-3]. These studies point out that EGFR mutation and its downstream KRAS mutation in the absence of inflammation forms much reduced tumor burden than when inflammation is present, supporting our hypothesis that there are two portions of tumor cells distinguished by the driven mechanism of replication, one being mutation-based self-driven and the other non-autonomous and the replication is dependent on inflammation induced by the self-driven tumor cells. One question remains to be answered is how to distinguish selfdriven and non-autonomous replication in a given tumor. Thus far, our empirical experiences have suggested that Ki-67 is the likely marker for self-driven replication whereas PCNA indicates replication by both self-driven and non-autonomous tumor cells. The identification of the presence of two populations of tumor cells and their connection by inflammation is critical for understanding a number of perplexing observations in the clinic situation. For example, the complete control by a drug targeting one specific mutation is dependent on this mutation being the only self-driven mutation in a given tumor, when more than one self-driven mutations are present, efficacy by any one drug would be short-lived for sure. This would explain the great variability between TKI efficacy in different cases. One way to tell whether a targeted therapy is likely to work well is to look at the ratio between the number of self-driven replicating tumor cells and the frequency of the mutation the drug is targeting. Only when the actively self-driven replicating tumor cells are less in number, than that of cells with the targeted mutation that the targeted therapy may work well (Figure 4). Otherwise the targeted therapy is imposable to achieve complete control of tumor replication due to the presence of other self-driven replication that is not controlled by the therapy. When most of the mutated cells are actively replicating, even when all of the self-driven replicating cells could be targeted by one therapy, the likelihood of this therapy being durable is unlikely because new mutations that bypass the targeted mutation are likely to develop during therapy due to leaky control. Even with tight control like the in this case, almost all targeted therapy cases develop drug resistance though development of non-targeted mutations. The pressing challenge, therefore, is to find ways to extend the control as long as possible. That requires the understanding of the question below.
Figure 4. Various situations under which targeted therapies may or may not work well.
The second question this case has brought forward that is seen in many TKI-treated cases is whether TKI therapy achieve efficacy through elimination of targeted tumor cells. This has been automatically assumed to be the case for many years as targeted therapy does bring tumor shrinkage that is a hallmark of tumor cell death. Previous study has also shown rapid death of tumor cells upon targeted therapy in animal model [4]. But the observations in this case argue against this assumption. It is expected that with 28% mutated cells in the tumor, an over twoyear treatment duration with initial tumor reduction and subsequent complete suppression of tumor growth would have eliminated most mutated cells that are capable of self-driven replication. If this is true, we should expect a slow rebound upon the brief TKI withdraw because only a few cells capable of rebound were left after the long period of effective TKI therapy. In contrast, we saw a rapid and sharp rebound of tumor replication and an escalating worsening of symptoms that support a full scale tumor replication by many cells. The around 30% of Ki-67 positive cells in the surgical tumor sample also support the presence of near total preservation of self-driven tumor cells after the long and effective TKI therapy that supposedly to eliminate most mutated self-driven tumor cells. These observations therefore point to an “inhibition”, but not “elimination” mechanism of tumor control. This lack of tumor cell killing by targeted therapy may explain why targeted therapy is rarely curable. Because self-driven tumor cells are not eliminated by the drug, there is no possibility of complete response by this therapy in this situation. In this case, like in many others taking similar therapy, the primary tumor shrank initially but then remained at a stable burden without further reduction in size. If we assume lack of direct killing of the targeted tumor cells, the reduction in tumor burden is therefore likely from the non-autonomous portion of the tumor. That suggests that many non-autonomous tumor cells probably die when factors driven their replication are withdraw (due to inhibition of inflammation induction by self-driven replication). One interesting issue relate to this lack of killing of the targeted tumor cells would be the relationship between disseminated individual latent metastasis and the drug that can inhibit its replication, thus establishment [5]. In as much as each disseminated tumor focus replicates independent of others, especially the primary tumor which contain a lot more cells than each metastasis, there is no reason to believe that targeted therapy fails to continue to control the replication of these foci when drug resistant cells develop form the primary tumor. A decision to switch or terminate a previously effective therapy is therefore a wrong choice to say the least [6]. Because these metastases are not killed but sure suppressed by the targeted therapy, a continued apply of this suppression is necessary and sometimes even critical for progression control. Yet we have seen many cases in the clinic that took the wrong turn following claim that a patient had developed resistance to a targeted therapy drug. These withdraws may have caused explosive development of previously well suppressed metastases [7]. It is for this reason that our patient was put on targeted therapy soon after surgery and will continue to be on the therapy even if subsequent drug resistant recurrence develops.
The third question this case has brought into focus is the relationship between tumor progression and symptoms. There was a tight association between tumor progression and symptom intensity. This type of tight association, especially the quick relief of symptom upon TKI therapy is a common observation in many cases under targeted therapy [8]. But this relationship has not been well explained in mechanism. It is always assumed that cancer symptoms are the result of tumor growth, despite in many cases where large tumor burdens have been identified with almost no severe symptoms. This disassociation between tumor presence and symptom has categorically been ignored by the main stream. From this case, the complete suppression of symptom when TKI therapy was in place for two years and the quick return of symptoms in days following drug withdraw accompanied by rapid rebound of tumor replication support the tight connection between tumor growth and symptoms. But tumor growth per sec should not be the direct reason of symptoms. Instead, the connecting factor is inflammation. As discussed above, replication of self-driven EGFR-mutated cells produce chemo taxis for inflammation which in turn drives the replication of the large number of non-autonomously replicating tumor cells. It is this inflammation that causes the symptoms. When self-driven replication is inhibited by TKI therapy, the source of inflammation is cut off and the symptoms are relieved quickly. Based on this explanation, we will be able to use the symptom as an indirect tumor marker to gauge the activity of self-driven replication, or use the relief of symptoms as a gauge for the effectiveness of therapy to suppress self-driven replication (thus the growth of the entire tumor). In fact, it is often the impression of the patients that the disease is under control when the symptoms subside in many cases and this impression is often correct. In as much tumor-induced inflammation may be systemic as measured by blood work in clinical tests [9], it is often confused with infection-induced inflammation and is the main reason antibiotics are abused clinically in cancer patients. It is highly likely that many of the wrong use of antibiotics in cancer patients suffering inflammation symptoms brought shortlived reduction in inflammation through killing gut flora, a mechanism that is not clear at the current time. Similarly, the blame for antibiotic as the culprit of failed checkpoint therapy may also need to be challenged based on the existence of broad clinical abuse of antibiotic in cancer patients suffering inflammation. It is likely the high inflammation and the lack of control for self-driven tumor cells behind the high inflammation that is the cause of failure for the antibody therapy to PD-1 as this type of therapy always cause a temporary tumor progression before re-control [10]. In the cases where there is no room for the temporary tumor progression, a hyper progression and accelerated death may take place before tumor control. Therefore, correctly recognizing the source of inflammation and symptoms in cancer patients could bring some beneficial manipulations that not only reduce the drive for replication of non-autonomously replicating portion of the tumor, but also help correct management of inflammation and avoid abuse of antibiotics in cancer patients.
The last issue related to this case that many other cases have also suggested is the origin of self-driven replicating tumor cells. This is rather a controversial issue because conventional wisdom has long believed that all tumor cells are self-driven. But with more précised genetic testing and clinical application, this claim seems incorrect in that many progressive tumor cells depend on some sort of host factors for growth. The best example is breast cancer cells expressing strong ER/PR receptors. They depend on female hormone for growth, thus suppression of these hormones with drugs post cancer surgery results in long-term recurrence control, so effective that it surpasses even immunity control in many solid tumors. In this case we have also witnessed inflammation-driven progression of non-autonomous replicating tumor cells that is common in lung adenocarcinoma. What is not clear, and partly revealed by this case, is whether self-driven mutations may generate during non-self-driven tumor replication? Or it comes from de novo mutagenesis of a normal cell that turns it into a cancer cell? The experience of this case argues for the former. At the initial diagnosis of adenocarcinoma by biopsy, genetic testing was performed with no EGFR mutations identified. After six rounds of chemotherapy and continued (or stimulated tumor progression, a second biopsy was carried out and the same testing service returned with identification of T790M mutation (28% frequency). Subsequent use of osimertinib confirmed this finding. If the first test is correct, this discrepancy can only be explained by assuming that the EGFR mutation emerged during chemotherapy. The origin of this mutation is likely to be from growing tumor cells that did not contain EGFR mutation. Development of cancer from trauma in some sarcoma cases and from pancreatitis in some pancreatic cancer has been repeatedly observed by us in the past decade (our unpublished observation). These cases point to a strong correlation between cell proliferation and cancer development. Considering that DNA mutation is only inherited during replication, placing the source of self-driven mutation to non-autonomous replicating tumor cells is a reasonable explanation. Based on this scenario, many clinical phenomena could be explained. For example, the most likely reason and source of drug resistance in TKI therapy would be leaky replication that generates new mutation during replication. In this consideration, two immediate predictions appear: 1) new mutation that not controlled by previous TKI therapy will likely come from the largest poor of tumor cells by chance, thus reduction of established tumor burden will significantly reduce this chance, and then lengthening control by current TKI therapy; 2) even with development of drug resistant mutation, individual disseminated tumor cells that are capable of self-driven replication will remain suppressed by current TKI therapy, thus despite of evidence of drug resistance, previously effective TKI therapy should be continued, not abandon. It was based on this thought; we prescribed surgery for prediction 1 and continued osimertinib regardless whether this case develops drug resistance subsequently for prediction 2. Future follow up of this case will test these predictions.
Although this case is all about the targeted therapy, the lessons from this case can be applied to other treatments as well. For example, antigen-specific antitumor immunity may target multiple tumor compositions depending on the available antigen from each part of the tumor. Based on observations in this case and many other similar cases where targeted therapy is highly effective, it can be deduced that the most effective antitumor immune response should be directed towards the selfdriven population, but not the inflammation-dependent nonautonomous population of the tumor. Immunity recognizing the metastasized tumor is more effective protecting post-surgery recurrence and metastases capable of forming a tumor and these tumor-forming metastases must be able to self-driven replication. Compare to targeted therapy with drugs, immunity is often without any side effects but the duration for effective protection may vary greatly from case to case depending on the strength and quality of the leftover immunity. The duration of protection by targeted therapy on the other hand is dependent on the likely hood of the development of a second mutation capable of selfdriven replication which should be very unlikely to happen if the drug is highly effective against individual disseminated. Tumor cells will harbor the targeted mutation. In this regard, targeted therapy should be more reliable in terms of holding metastasis on check than immunity because it never decays as immunity would. Based on this rationale, in cases where a tight tumor control is achieved by targeted therapy, removal of all visible tumor burdens by ways of various modalities such as radiation and surgery and leaving the protection of recurrence to targeted therapy should be a wise choice. It is not the presence of metastasis that is critical to long term survival, it is whether there is a reliable way to control the metastases that is more important. In a number of cases of effective antitumor immunity, even stage IV cancer can be eradicated and kept for long term disease-free survival (our unpublished results).
Citation: Tusung K (2019) The Presence of Autonomous and Non-Autonomous Replication in Cancer and their Connection through Inflammation: Lessons from one Lung Cancer Case. J Clin Exp Dermatol Res. 15:171. doi: 10.35248/1745-7580.19.15.171
Received: 21-Oct-2019 Accepted: 05-Nov-2019 Published: 11-Nov-2019
Copyright: © 2019 Tusung K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : none