Forest Research: Open Access
Open Access
ISSN: 2168-9776
ISSN: 2168-9776
Research Article - (2015) Volume 4, Issue 1
Hydrologic, chemical and physical factors controlling P export from 10 livestock farms located in Wensleydale were considered in order to estimate the risk of P loss to surface waters in a Non Nitrate Vulnerable Zone (non NVZ). Phosphorus source factors were obtained from a farm investigation questionnaire as well as from soil analysis results. Transport factors were examined using a GIS as a tool to implement a predictive model of delivery of P to watercourses. The classification of grassland into different risk categories was carried out using the Pennsylvanian P Index in a GIS. Finally, this risk assessment has yielded that non NVZ areas can pose a risk of breaching the Water Framework Directive (WFD) objectives. A wide range of mechanisms including voluntary schemes are needed in order to reach good ecological status of freshwaters by 2015 in the UK.
<Keywords: NVZ; Phosphorus Index (PI); GIS; Water framework directive; Livestock farming
Several published studies have demonstrated the impact of diffuse agricultural pollution on rivers ecological functions as a result of poor water quality [1-4].
The increase use of fertilisers in order to increase production efficiency in farming systems has been pointed out as the main factor that leads to nutrient emissions to freshwaters [5,6]. In this regard, agricultural stewardship alongside physicochemical soil properties, geomorphology or even weather, are other factors that may contribute to increasing nutrient water pollution [7,8].
Nitrogen (N) and Phosphorous (P) have been widely studied due to the important role they play on water eutrophication [9,10]. This excessive enrichment of water with N and P leads to algal blooms and hence, anoxic conditions of receiving waters that have proven to be harmful to aquatic life [9,11].
In spite of the fact that P is a nutritional limiting factor for algal growth [12,13] that can substantially increase the risk of eutrophication in waterbodies, more attention was paid to N due to the EU Nitrate Directive. This makes sense if we focus on the quality of drinking water, as some human health problems such as methemoglobinemia are associated with high nitrate concentrations [6,14]. Furthermore, N is more likely to leach with rainwater moving through the soil [5] what brings about lower nitrate retention by soil and hence, a higher nitrate concentration in freshwaters. By contrast, high concentrations of P in freshwaters are difficult to find as phosphate anions are mostly retained by soil particles [15]. All these factors were mainly considered when MS including the UK, were required by the Nitrate Directive to identify nitrate pollution in freshwaters [16].
The designation of NVZs (Nitrate Vulnerable Zones) depends on nitrate concentration exceeds the threshold value of 11.3 mg N l -1 in freshwaters [14]. In England 68 of the territory has been designated as NVZ [17]. This implies a set of measures that farmers in NVZs are obliged to implement in order to minimise water pollution. In the case of England, farmers in NVZ are required to meet the Nitrate Pollution Prevention Regulations 2008 (Statutory Instrument 2008/2349).
According to this regulation, all farmers must have a planning for N use where they can show the amount of N fertiliser that they need to apply. This should be based on N content of organic fertilisers, this is Farm Yard Manure (FYM), slurries, or both, as well as Soil Nitrogen Supply (SNS). Some of these requirements differ depending on the type of farm. For example pig farms are required to provide storage for organic manures for at least 6 months (1 October to 1 April inclusive) whilst dairy farms need 5 months (1 October to 1 March inclusive). This is fundamentally based on estimations of the nutrient content of organic manures, as Table 1 shows.
| Dry matter (%) | Total Nitrogen (Kg N/m3) | Available phosphate Kg P2O5/t | |
|---|---|---|---|
| Cattle | 6 | 2.6 | 0.6 |
| Pig slurry | 6 | 4.4 | 1.3 |
Table 1: Nutrient content of slurries depending on the type of farm [34].
Furthermore, there are restrictions for spreading livestock manure as farmers cannot exceed the amount of 170 kg ha-1 of total N per year. There are also spreading restrictions, mostly in the winter period depending on the type of land. In the case of grassland, spreading of organic fertilisers is restricted between September and January depending on soil characteristics. Similarly, spreading mineral fertiliser is not permitted between the 15th Sep and 15th Jan. Further restrictions include for example, not spreading manure within 10 m. from surface waters as well as not spreading when soil is waterlogged, snow covered or frozen.
However, farms located in the remaining 32 of the English territory, this is non-NVZs, are not required to comply with these rules.
Nevertheless, storage for organic manures is regulated in all the territory by the Water Resources (Control of Pollution) (Silage, Slurry and Agricultural Fuel Oil) (England) Regulations [18]. The application of this regulation brings about that every livestock farm in England must provide enough slurry storage capacity for the maximum amount of slurry “likely to be produced in any four month period”. This rule aims at reducing the number of organic fertiliser applications in order to reduce the risk of nutrients loss to freshwaters, in cold seasons characterised by the low nutrient uptake rates by vegetation [19].
Nonetheless, there is a gap in this regulation as storage systems built for that purpose before 1991 are excluded. According to Figure 1, over 70 of slurry storage systems in areas not included in NVZ, date back more than 10 years and hence, many of them could have been constructed before 1991.
With the advent of the WFD in 2000, freshwaters in the UK including the non-NVZs, should meet good ecological and chemical status by 2015 [20,21]. This framework brought about The England Catchment Sensitive Farming Delivery Initiative (ECSFDI) in order to meet the WFD objectives. This Iniciative tries to raise awareness and deliver advice among farmers for reducing nutrient water pollution [14]. In this regard, the ECSFDI is a project where is up to farmers to get involved, unlike farms in NVZ where they are required to apply the Code of Good Agricultural Practice [17].
Moreover, some recent studies undertaken to determine the effectiveness of NVZ and ECSFDI have indicated the lack of evidence of significant water quality improvements in the UK [14,22]. In fact, Worral [22] questioned the effectiveness of measures that mainly aim at reducing fertiliser inputs in farms included in NVZ, whereas other factors such as connectivity of nutrient sources and hydrological flow paths can be determinant for nutrient pollution at a catchment scale. In this sense, the catchment-based approach introduced by the WFD is crucial to reduce diffuse water pollution, as farms in non-NVZ may pose a risk of failing good status objective.
Assessment Methods
As stated above, P has been pointed out by numerous authors [10,23-25], including the WFD, as a key nutrient for addressing water eutrophication derived from diffuse nutrient loss from agriculture. However, this is a hard task considering the complexity of the processes involved in the delivery of P to freshwaters [15].
Due to time constraints to meet WFD targets, consistent approaches should be implemented in order to assess the risk of nutrient pollution posed by agricultural systems. This information would be useful for identifying P emission hotspots and hence, increasing the effectiveness of actions to mitigate diffuse pollution [26].
Suitability of assessment methods will vary according to the target [27]. For example, the measurement method could provide an accurate assessment of nutrient delivery at a catchment scale as long as analytical equipment was available in representative sample sites [23]. Unfortunately, this is not always possible as monitoring is expensive and time consuming.
Moreover, the nutrient budgeting method has proven useful to estimate nutrient surpluses in soil considering inputs such as animal feed, internal cycling and outputs [28]. Domburg [29] demonstrated higher P surpluses were linked to livestock farms mainly due to fertiliser inputs. Similarly, Edwards [30] published that higher P surpluses were associated with grassland farming systems (Table 2). In this sense, other studies have yielded that a common practise among farmers is to apply fertiliser on the basis of N, not considering concentrations of P already present in the soil [31,32]. Thus, this information can contribute to optimising farm management, in particular nutrient management.
| Farmcategories | Land area (%) | P surplus (%) |
|---|---|---|
| Dairy | 19 | 29 |
| Cropping | 41 | 31 |
| Pigs/poultry | 1 | 17 |
| Remaining | 39 | 23 |
Table 2: Contribution of various farm categories to the annual total P surplus for the UK, expressed as a proportion of total agricultural land. [30].
However, some additional considerations appear when the target is to assess the risk of P loss from grassland farming systems to freshwaters. Source factors are important in order to estimate nutrient availability such as P availability, yet delivery of P is conditioned by transport factors [27]. In fact, “transport factors are what transform potential P sources into actual P losses from a field or watershed” [33]. Hence, a risk assessment method is selected in this study in order to determine the potential for P loss from livestock farms in a non NVZ. The specific objectives of this research are to i) ascertain organic fertiliser management on livestock farms on a non NVZ area, ii) determine if there is a relationship between P application to land and soil P index, iii) produce a P risk map for the study area, and iv) ascertain the risk of failing the WFD objectives in non-NVZ area.
Study area
The current study was undertaken in the Upper Wensleydale catchment, within the Yorkshire Dales, in North Yorkshire (Figure 2). The most important urban areas located near the study area are Askrigg, Bainbridge and Hawes. All this area has a long tradition of livestock farming according to the Yorkshire Dales National Park Authority.
The main waterbodies included in the study area are River Ure, River Bain and Semerwater Lake. It should be highlighted that Semerwater Lake has many inlet streams as it is the largest glacial Lake within the Yorkshire Dales. Many of these inlet streams are found in a small valley called Raydale and cross most of the farm fields included in this research. The lowest point in the study area is adjacent to the River Ure at 205 m whilst the highest elevation rises to almost 600 m. Moreover, it was observed that the annual precipitation data from July 2011 to June 2012 presented a significant variation between gauge stations that are only 8 Km apart. In this sense, the gauge at Hawes (227 mAOD) shows a total annual precipitation of 1599 mm whilst the gauge at Moorland Cottage (343 mAOD) was 2502.1 mm. This shows that it rains more at higher elevations. Furthermore it shows that the study area is a very humid region where soils are predominantly acidic.
Farms characterization
The types of livestock farms included in the study were mostly dairy and sheep farms (Table 3). Furthermore, different types of farms may bring about differences in the risk of P loss to watercourses. Information associated with livestock numbers, slurry storage systems and farm management was collected from a Farm Investigation Questionnaire (Appendix). Farm level data on the organic and mineral fertiliser rates to grass as well as timing of spreading was also obtained from the questionnaire. In fact, this data were different from the figures shown in The British Survey of Fertiliser Practice [17] where the five-year mean for grazed grass was 10 kg ha-1. The N:P:K mass ratio of mineral fertiliser applied to land was used to obtain the application rate of P (kg ha-1) considering that P fertiliser is present as P2O5 (56.4 oxygen and 43.6 elemental phosphorus). Similarly, the available P from organic fertiliser (manure/slurry) applied to the soil in some farm fields, was obtained from the nutrient analyses made by the ECSFDI. On the other hand, for those farms that an analysis report from ECSFDI was not available, data for P concentration in slurries/manure were obtained from the Fertiliser Manual (RB209) [34]. In this regard, no overall quantification of the accuracy of the calculations could be given.
| Farm Id | Sample Id | Type of livestock |
|---|---|---|
| 1 | 1B | Dairy and sheep |
| 1 | 1T | Dairy and sheep |
| 2 | 2S | Dairy and sheep |
| 3 | 3S | Dairy |
| 4 | 4S | Beef, sheep and lamb |
| 5 | 5S | Dairy and sheep |
| 6 | 6S | Beef and sheep |
| 7 | 7S | Dairy, sheep, lamb and beef |
| 8 | 8S | Beef and sheep |
| 9 | 9S | Dairy and sheep |
| 10 | 10S | Dairy, sheep and lamb |
Table 3: Identification of farms and composite soil samples included in the study.
These data were included in the GIS model to calculate the average application rate of P per field block.
The livestock farms included in this study extend to 2014 hectares of meadow, inbye pasture, woodland and moorland. The farm fields comprise two main land uses; rough grazing that includes moorland (70) and improved pasture (30 ).
Also relevant to this study is the livestock units per hectare (LU/ Ha) as it is well known that animal trampling is a factor that increases the compaction of the soil and hence, brings about an increase of runoff volumes [35]. LU/Ha was estimated depending on the type of land use. Moorland is normally used for grazing sheep but not hardy cattle. In this respect, rough areas will have lower LU values compared with improved areas. The values are obtained in the GIS by dividing the correspondent number of livestock units by the area (in hectares), considering the length of time the stock are kept on the area. In this study, we considered that cattle are kept indoors between October and April. In addition to this, a table of ratios based on feed requirements of different livestock [36] was used for these estimations.
Soil sampling
The timing of the farm visits coincided with a period of very wet weather by the middle of July. Soil cores were taken from the top 7.5 cm. according to Rowell [37] recommendations for grassland. A composite soil sample for each study farm was made up of 3 individual cores taken at sites close to surface waters (less than 50 metres). This sampling aimed to obtain soil P concentration in areas that pose a higher risk due to its proximity to watercourses (Figure 3). The exception was Farm 1 as one more composite soil sample (1B) was taken from a field where dairy cattle were trampling on the river bank.
Soil Analysis
In the field, soil texture was assessed by hand following a guide for mineral soils taken from Rowell [37]. On return to the laboratory, soil moisture content was determined gravimetrically after oven-drying soil samples overnight at 105°C. The loss-on-ignition method was used to estimate the soil organic carbon using the procedure presented in Ball [38].
Soil pH was measured by placing a glass electrode in a mixture of soil and deionised water
Furthermore, pH was also measured in a salt solution in order to release “reserve” acidity of colloid surfaces.
Olsen’s P was determined according to the method presented in Rowell [37]. Soil samples were dried at 40°C for 48 hr. Subsequently, they were crushed in a porcelain mortar to break up large aggregates and sieved using a 0.2 mm stainless steel sieve. 3 reagent blanks were extracted in order to determine if phosphorus was present in the reagents. Due to high P concentrations in the soil extracts, the absorbance was greater than the top standard and therefore the amount of extract/sulphuric acid had to be reduced in order to match the standards.
Statistical Analysis
Pearson’s correlation tests were undertaken in Minitab v16 (Minitab Inc., State College, PA, USA) and considered significant where P<0.05. These analyses were conducted to assess any linear relation between P concentration in farm soils and farm practices.
Risk mapping
All the research was built on a predictive model carried out using ArcGIS V.10. Risk areas for P losses from field blocks were mapped using the Pennsylvanian P Index as it is well documented (Table 4) [39].
| Part A- Screening tool | |||||||
| Evaluation Category | |||||||
| Soil Test P | >200 mg P kg -1 | If yes to either factor then proceed to Part B | |||||
| Contributing Distance | <45 m. | ||||||
| a Contributing Distance> 45m. AND field artificially drained |
|||||||
| Part B – Source factors | |||||||
| Soil test | Soil Test P (mg P kg -1) | ||||||
| Soil Test P Rating = 0.20* Soil Test P (mg P kg -1 ) | |||||||
| Fertiliser P rate | Fertiliser (mg P kg -1) | ||||||
| Manure P rate | Manure (mg P kg -1) | ||||||
| P source application method | 0.2 Placed or injected 5cm or more deep |
0.4 Incorporated <1 week |
0.6 Incorporated>1 week or not incorporated April – October |
0.8 Incorporated>1 week or not incorporated Nov – March |
1.0 Surface applied to frozen or snow covered soil |
||
| Fertiliser Rating = Rate × Method | |||||||
| Manure P availability | 0.5 Treated manure/Biosolids |
0.8 Dairy |
1.0 Poultry/Pigs |
||||
| Manure Rating = Rate × Method × Availability | |||||||
| Source Factor = Soil Test P Rating + Fertiliser Rating + Manure Rating | |||||||
| Part C – Transport factors | |||||||
| Erosion | Soil Loss (tonnes ha-1) | ||||||
| Runoff potential | 0 Very low |
2 Low |
4 Medium |
6 High |
8 Very high |
||
| Sub-surface drainage | 0 None |
1 Some |
2b Patterned |
||||
| Contributing distance | 0 >150 m. |
2 150 to 100 m. |
4 100 m. to 75 m. |
6 45 to 75 m. |
8 <45 m. |
||
| Transport Sum = Erosion + Runoff Potential + Sub-surface Drainage + Contributing Distance | |||||||
| Modified Connectivity | 0.7 Riparian buffer – applies to distance<45 m. |
1.0 Grassed waterway or none |
1.1 Direct connection – applies to distance>45 m. |
||||
| Transport Factor =Modified Connectivity × (Transport Sum/22) | |||||||
| aModification introduced from the Danish P Index bOr rapid permeability soil near a stream |
|||||||
| Phosphorus Index Value = 2 × Source Factor × Transport Factor | |||||||
Table 4: The Pennsylvania P Index [39].
Source factors: All source factors were estimated using the data listed above. Data on soil test P (Olsen Method) was obtained from the analysis results for each farm. The different fertiliser and manure application rates, depending on the farm, between April to October and November to March, brought about two different P risk estimations for each farm. This is PIV_S (Phosphorus Index Value_April to October) and PIV_W (Phosphorus Index Value_November to March).
Transport factors: Soil erosion (tonnes ha-1) was calculated by the Revised Universal Soil Loss Equation (RUSLE) in a GIS in order to create a model to obtain the annual soil loss for each farm holding. The model was created according to Shiferaw [40] and is based on the RUSLE equation (1).
A= LS* R* K* C* P (1)
Where:
- A is the annual soil loss (metric tons ha-1yr-1);
- LS = slope length factor (dimensionless); this was derived from a 5 m. resolution DEM where local depressions (sinks) were previously removed. The equation (2) given by Morgan [41] was used to estimate the LS factor.
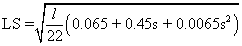 (2)
(2)
Where:
l = slope length in m.; it was obtained by using the ArcHydro tool “Flow Length”.
s = percent slope; it is the result of using the Spatial Analyst tool “Slope”.
- R is the rainfall runoff erosivity factor [MJ mm h-1 ha-1 yr-1]; Due to the lack of rainfall intensity data as well as storm kinetic energy, R was derived from mean annual and monthly rainfall data obtained from the previously mentioned gauge stations (Table 5).
| Gauge Station | 2011 | 2012 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jul | Aug | Sep | Oct | Nov | Dec | Jan | Feb | Mar | Apr | May | Jun | |
| Hawes | 118 | 158 | 141 | 170 | 78,8 | 238 | 154 | 84,8 | 23,8 | 148 | 97,6 | 187 |
| Moorland Cottage | 125 | 244 | 273 | 241 | 160 | 428 | 165 | 220 | 51,1 | 151 | 127 | 317 |
Table 5: Monthly rainfall data for the study area. (Environment Agency).
- C is land cover and management factor (dimensionless, ranging between 0 and 1); According to Shiferaw [40] the C-Value for grassland is 0.01.
- P is conservation practice factor (dimensionless, ranging between 0 and 1). In this study we considered P = 1.
The relationship between rainfall data and altitude was expressed in a linear equation in order to interpolating the rainfall values across the study area. A 5 m. resolution DEM was used along with the derived equation to obtain the mean annual rainfall (MAR) raster. Fournier Index (3) was obtained according to Arnoldus [42]:
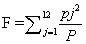 (3)
(3)
Where, F = Fournier Index;
pj is the monthly rainfall for month;
P is the annual rainfall
F was linked to R through the empirical formula given by
R = (4.17F – 152)/17.2 [42].
K factor was obtained by using the following equation [43]:
K = 2.1 × 10-6 × M1.14(12-OM) + 0.025 (S-3) + 0.0325 (P-2)
Where;
K = soil erodibility factor in t.h/MJ.mm; this layer is based on data such as soil texture and percentage of organic matter, obtained from the analysis results.
K factor was obtained by using the following equation [43]:
M = (Percentage very fine sand + Percentage silt) x (100 – Percentage clay)
OM = Percentage of organic matter obtained from the soil samples.
S = Code according to the soil structure (very fine granular = 1, fine granular = 2, coarse granular = 3, lattice or massive = 4);
P = Code according to the permeability/drainage class (fast = 1, fast to moderately fast = 2, moderately fast= 3, moderately fast to slow = 4, slow = 5, very slow = 6). This information was obtained from Soilscapes viewer.
Table 6 shows the different textures found in farm soils (all of them histosols).
| Sample Id | Textural class | M | OM (%) | S | P | K |
|---|---|---|---|---|---|---|
| 1B | Sandy Loam | 8100 | 6.14 | 3 | 3 | 0.38 |
| 1T | Sandy Loam | 8100 | 3.64 | 3 | 3 | 0.53 |
| 2S | Sandy Loam | 8100 | 4.77 | 3 | 3 | 0.47 |
| 3S | Silty Clay Loam | 4900 | 6.14 | 2 | 5 | 0.27 |
| 4S | Clay Loam | 4900 | 6.09 | 3 | 4 | 0.26 |
| 5S | Silt Loam | 8100 | 6.30 | 2 | 4 | 0.38 |
| 6S | Loam | 6400 | 5.43 | 3 | 2 | 0.30 |
| 7S | Loam | 6400 | 4.78 | 3 | 2 | 0.33 |
| 8S | Loamy sand | 10000 | 6.03 | 3 | 1 | 0.42 |
| 9S | Sandy Clay Loam | 6400 | 4.76 | 2 | 3 | 0.34 |
| 10S | Sandy Loam | 8100 | 3.74 | 3 | 3 | 0.53 |
Table 6: Values of the parameters used to obtain K for each farm.
Runoff potential
The relationship between potential runoff and soil moisture has been demonstrated in different studies [44,45]. The Topographic Index model [46] was implemented in this study in order to establish different thresholds for runoff potential. Threshold values for classifying areas where saturation excess overland flow is likely to occur, were based on field work as well as the waterbodies layer derived from the 1:10,000 topographic map. These values were finally reclassified according to the Pennsylvania P index.
The Topographic Wetness Index (TWI) was calculated as follows [47]:
Wi = log (a / tan ß) Where,
a is the upslope contributing area per unit contour length (cell size * flow accumulation) and tan ß is the local slope gradient for estimating a hydraulic gradient (ß is the slope converted in radians). In addition, flow accumulation layer was derived from a DEM where sinks were removed and null (No Data) cells were filled using focal function (e.g. ‘mean’).
It was assumed that wetter cells have more soil moisture and hence, more likely to induce saturation excess overland flow.
Furthermore, values were transformed to the same unit of measurement scale. This was done on a 0 to 1 scale using this generic formula (4).
(“Oldgrid” – minimum value) / (maximum value – mininmum value) (4)
Finally values were reclassified according to Table 7.The values used for “sub-surface drainage” were based on the farm visits.
| Old value | New value |
|---|---|
| 0-0.06 | 0 |
| 0.06-0.18 | 2 |
| 0.18-0.3 | 4 |
| 0.3-0.41 | 6 |
| 0.41-1 | 8 |
Table 7: Reclassification values for TWI.
The contributing distance was calculated in GIS by appending the waterbodies raster layers (rivers, streams and lakes) in order to obtain a cost distance raster. In this regard, the flow direction raster was used as the input cost distance raster, as water always flows down a slope. Finally, distance values were reclassified according to Table 4.
Sub-surface drainage information was provided by the Yorkshire Dales National Park Authority.
The connectivity values were based on the analysis of highresolution aerial images. Therefore, values were specific for each field block.
Despite the fact that all the farms are not in a NVZ area, 90 accepted to receive advice from the ECSFDI according to the WFD objectives. However the farms management practices could substantially be improved according to the data collected during the farm visits.
Fertiliser intputs
Data for fertiliser inputs, this is mineral as well as organic fertiliser, were crucial to determine the risk of P loss when the P Index assessment was carried out (Table 8).
| Sample Id | P (ppm) | Soil Test P (ppm) | P availability Index (extractable phosphate ppm of soil) | P Index(ADAS) | ADAS Interpretation | N:P:K Mass ratios | Mineral F. application rate (kgN ha-1) | Total P (FYM+Slurry) application rate (kg ha-1) |
| 1B | 73.62 | 14.7 | 71-100 | 5 | Very high | 34:20:10 | 40 | 8.18a |
| 1T | 33.97 | 6.8 | 26-45 | 3 | Medium to high | No chemicals | - | 8.18a |
| 2S | 81.98 | 16.4 | 71-100 | 5 | Very high | 25:05:05 | 170 | 8.4 |
| 3S | 79.29 | 15.9 | 71-100 | 5 | Very high | 25:05:05 | 250 | 19.2 |
| 4S | 60.13 | 12 | 46-70 | 4 | High | 20:10:10 | 50 | 23.75 |
| 5S | 37.21 | 7.4 | 26-45 | 3 | Medium to high | 25:00:14b | 125 | 17.3a,c |
| 6S | 46.65 | 9.3 | 46-70 | 4 | High | 20:10:10 | 123 | No data |
| 7S | 91.69 | 18.3 | 71-100 | 5 | Very high | 24:00:15 | 250 | 49.3d |
| 8S | 38.82 | 7.8 | 26-45 | 3 | Medium to high | 20:10:10 | 250 | 23.75 |
| 9S | 44.76 | 9.0 | 26-45 | 3 | Medium to high | 20:10:10 | 125e | 27.58 |
| 10S | 25 | 5 | 16-25 | 2 | Slightlylow tomedium | No data | - | 7.66a |
| aNo data for FYM application rate bThis mass ratio corresponds to April. 92.5 kgNha-1of urea (48% N) are applied in March, whilst 92.5 kgNha-1(24:00:14) are applied in June. c17.3 corresponds to the slurry application rate in spring, whilst 11.5 is the slurry application rate in winter. d49.3 corresponds to the manure (FYM&slurry) application rate in spring, whilst 45.4 is the figure for winter. e125 corresponds to the application rate over all the field blocks, except for moorland where does not apply and meadow where the mineral F. Application rate is 500kgN ha-1 (20:10:10). |
||||||||
Table 8: Fertiliser application rates and P availability indices (ADAS) for the correspondent soil samples.
The procedure followed by farmers to estimate the manure application rates was basically to avoid “smothering” the grassland. Only 20 of the farmers assured that manure application rates varied depending on the season (applying higher rates in spring). All the farmers provided data for slurry application rates, unlike data for FYM where 40 of the farmers did not have figures for it. This posed a problem for the risk assessment of farms 1, 5, 6 and 10.
Information about soil analysis and nutrient content of manure deserve special attention. In this regard, 30 of the farmers assured to make soil analysis, normally every 3-4 years, whilst Farm 5 also measured the nutrient content of manure and the P concentration of soil. This information links to the mineral fertiliser mass ratio, as no P is applied to soil in Farm 5. Regarding the rest of the farms, estimations of mineral fertiliser application rates were made on the basis of N and varied widely among them.
Moreover, it should be highlighted that some of the farms received between 1 and 3 years ago, from the ECSFDI a Nutrient Management Plan. This report was built on a soil nutrient analysis as well as on standard figures from the RB209 Fertiliser Manual in order to advise farmers about the fertiliser application rates. However, the results for P concentration obtained from soil analysis in the laboratory for each farm, were generally high and even very high. In this regard, Table 8 shows that over 50 of the results are included in high and very high categories according to the P availability index used in Britain by ADAS. This means that soils are so enriched with P.
Pearson’s correlation tests showed no significance relationship (P < 0.05) between P application rates and P Index. This could be due to the lack of accurate and complete data for the P application rates. In this respect, special attention deserves farm 7 as it obtained the highest P concentration value despite no mineral P was applied this last year according to the farmer. However, the sum of available P from slurry and FYM results in the highest value. This may link to some studies that highlighted that farmers normally undervalue the N:P:K content of slurry and FYM [31,32].
Slurry storage and timing of application
The information provided by the farmers shows that aspects associated with slurry storage as well as the timing of manure spreading, are far from meeting the Code of Good Agricultural practices required for farms in NVZ areas. Similarly to the figures for slurry store construction [48], 90 of the study farms had slurry storage systems built before 1991. Therefore, they are not obliged to meet the SSAFO requirements for providing slurry storage capacity for a four month period. This information is closely related to the manure application frequency. In fact, 60 of the farms spread manure, at least, once per month during the winter period.
The factors mainly considered by farmers when spreading are associated with wet soil conditions as well as storage capacity. All of the farmers assured that they do not spread manure when is raining or soil is too wet, as this may cause a loss in traction for the tractor as well as soil erosion. However, it should be stressed that all of them spread manure yet soil is snow covered or frozen.
The reasons given by farmers are the better traction for the tractor as well as the limited slurry storage capacity.
The information above shows that limited capacity to store manure exerts a strong influence on the frequency of its applications. This makes sense if we take into account that 40 of the farms only had slurry storage capacity for a maximum of 6 weeks, and only 20 can provide slurry storage capacity for a four month period. It is not surprising then, that, 70 of the farmers would be willing to change the frequency of spreading manure in case of larger capacity to store it.
On the other hand, the rest of the farmers maintain that spreading large amounts of manure during a short period (after January) could be counterproductive, as livestock start
grazing in spring. However, Hooda [49] demonstrated that limited slurry storage capacity and hence, slurry application during winter months is linked to high P concentrations in drainflow. In spite of this, it is discouraging that 90 of the farmers do not consider increasing the capacity to store manure. Among the reasons given are the milk price drop and the lack of grants for this issue.
Application method
Another aspect is the method used to spread manure. This is one of the P source factors considered in the P Index assessment. Slurry application in all the field blocks is carried out with a splash plate system whilst FYM is spread using a manure spreader. Only Farm 5 used the shallow-injection technique to spread slurry in spring (Figure 4). These data show the lack of effective techniques for application of manure what results in an increase of the risk of P loss according to the P Index assessment. The reason for this is associated with the exposure of manure to precipitation events and subsequent nutrients loss in runoff [50,51].
Figure 4: Slurry application methods “(a) on top of herbage and over entire spreading width with splash-plate method; (b) in lines on top of herbage with band-spreader method; (c) in lines below herbage, but above the soil surface, with the trailingshoe method; and (d) below the soil surface (approximately 5 cm) with the shallow-injection method”[51].
Livestock density
The LU/Ha values were most of them similar or lower in comparison with the average stocking density over all lowland forage area in England (0.58) [52]. Only the result for Farm 2 almost reached 2 LU/Ha. According to Chesterton [52] 2 LU/Ha is considered to be a problem in a NVZ area. In fact, Edwards [30] pointed out that livestock numbers is an important factor contributing to the variation of P surplus. Nevertheless, Pearson’s correlation tests showed no significance relationship (P < 0.05) between P Index and LU/Ha.
Mitigation measures
The effectiveness of some agricultural stewardship measures for ameliorating nutrient water pollution have been demonstrated across numerous studies [31,35,53,54]. Regarding possible measures implemented to reduce the risk of nutrient loss to freshwaters, 40 of the farms had fenced off its waterways. Thus, cattle had access to waterbodies in 60 of the farms what poses a risk for water quality. As stated above, sample 1B was taken from a near river bank trampled by dairy cattle. The impact of cattle poaching around river banks was stressed by Walling [55] as an important sediment source that links to P loss to watercourses.
In addition to this, 60 of the farmers spread fertiliser within 5 to 6 meters of watercourses where there are no riparian buffers. This spreading distance from watercourses considering the no existence of riparian buffers could be clearly insufficient to ameliorate the risk of nutrients reaching the surface waters. In this regard, Heathwaite [31] highlighted the small removal efficiency of Total Phosphorus (TP) using a 10 metre buffer strip, when organic fertilisers are applied.
Mapping of risk mapping
The implementation of the Pennsylvania P Index on the study farm holdings resulted in large areas being classified as “no risk”. In particular, 80 of the farms had more than half of the grassland being assigned a value of 0. These field blocks correspond to rough grazing where most of it is moorland. Farmers assured not to spread fertiliser on this land types, from what they were considered to have a Soil Test P <200 mg P mg-1. This information was taken into account when soil sampling was carried out. Therefore, no soil samples were taken from those land types. The consequence of this was that “no spreading areas” scored a value of 0 for Soil Test P rating (Figure 3). The remaining field blocks were mostly classified as having a low risk of losing P to freshwaters (Table 9).
| P Index Value | Rating | % of land(PIV_W) | % of land(PIV_S) |
| 0 | No risk | 67.25 | 67.25 |
| <60 | Low risk | 23.14 | 22.26 |
| 60-80 | Medium risk | 4.39 | 2.86 |
| 80-100 | High risk | 2.11 | 2.07 |
| >100 | Very high risk | 3.12 | 5.57 |
Table 9: General interpretation of the Pennsylvania P Index [39] and total % of land in each risk category. Calculating the Pennsylvania P Index on the farm holdings located in Wenslaydale.
Moreover, the application of mineral fertiliser only in spring as well as different amounts of manure applied by some of the farmers depending on the time of year, were taken into account in order to classify the surface area of each farm in risk categories (Figures 5, 6 and 7a).
Although Table 9 shows the overall figures for each risk class, it is recommended to observe Figure 5. In this regard, P Index for farms 1 and 2 did not differ between the two studied periods (PIV_W & PIV_S). The reason is that the small amount of mineral P fertiliser applied in spring does not result in more areas included in higher risk categories. Furthermore, these two farms maintain the same manure application rates during the two study periods.
Another group is formed by farms 4, 5, 7 and 10. Figure 5 for these farms, shows slight increase of areas included in higher risk categories during the PIV_W period.
These farms have in common the small amounts of mineral P fertiliser applied in spring (Table 8). The most obvious PIV increase occurs in farm 7 due to the high application rate of manure in winter. Although the amount of manure applied to soil, is even slightly higher in spring, the Pennsylvania P Index considers the risk of P loss is 40 less between April and October. This is the reason why adding fertiliser in spring may not pose a higher risk of P loss in this time of year. The opposite effect occurs on farms 3, 6, 8 and 9, where there is a higher risk of P loss during the PIV_S period. In this respect, farms 8 and 9 deserve special attention as the increase of surface area in the very high risk category (>100 according to P Index Rating) is dramatic. The reason seems evident if we look at the figure for mineral P application rate. In this sense, these two farms have the highest inputs of mineral P. This substantial increase of P inputs can clearly be observed if we compare Figures 6 and 7a. The red area indicating “very high risk” is much larger in Figure 6, for both farms. Furthermore, the results shown on the maps represent complex information through a very intuitive visualization. For example, Farm 9 can pose a high risk of P delivery to Semerwater Lake.
Moreover, the P risk maps obtained for each farm are not only the result of fertiliser application rates and soil analysis results, but also transport factors which magnitude varies in each area or pixel. The importance of transport factors in the risk maps, can be observed if we take into account that farms with higher figures for “Soil Test P” are not necessarily the ones with larger areas in high risk categories.
In particular, soil loss values or soil erosion greatly influences the risk category of each pixel. The use of a 5 m. resolution to obtain the different factors included in the RUSLE equation, guarantees accurate results.
Nevertheless, the rainfall runoff erosivity factor obtained for the study area could be improved if rainfall intensity data had been available. The use of the empirical formula given by Arnoldus [42] could have increased the systematic error of the model due to the use of statistical techniques to estimate R [56].
However, the use of Fuzzy methods was crucial to represent the continuous properties of soil as well as the geomorphology in order to add as much detail and information as possible [57]. On the other hand, the maps are also the result of using Boolean methods in order to include values associated for example with sub-surface drainage.
Landscape connectivity
To meet the objectives of the WFD, at the catchment scale, requires identifying the sources of diffuse pollution on small tributaries [58,59]. Thus, the approach taken in this study was to determine the degree of risk posed by livestock farms in a sub catchment, classified as non NVZ. This classification based on the Nitrate Directive, set clear boundaries to ensure that regulation resources focus on areas where nitrate pollution is known to be a problem (NVZ).
However, some studies have questioned the effectiveness of these policies, specifically the Nitrate Directive [14,22], as they do not consider the upstream-downstream linkages, particularly the nutrient spiralling concept. In this regard, River Ure rises very close to the study area and then, flows eastward through a NVZ. This river is the major pathway for the transport of nutrients from the study area to NVZs. Meybeck [60] in a study carried out in the Seine river basin, demonstrated a continuous rise in P concentrations downstream. Thus, important nutrient loadings may result from the Critical Source Areas (CSA) identified in the study farms and reach these NVZs. This shows that water pollution problems should not exclusively be identified from concentration data such as the threshold value of 11.3 mg N l -1 in freshwaters, established by the Nitrate legislation. Water eutrophication in lakes has been demonstrated to occur depending on the N:P mass ratio [30]. The appropriate method should then quantify nutrient loadings or flux rates coming from point and non-point sources of pollution [61], although the bioavailability of P fractions in freshwaters and the time of year (spring/summer) are major factors that influence the risk of water eutrophication in flowing waters [10,62].
Managing source factors
The ECSFDI has been the main mechanism in England that aims at reducing nutrient loadings at the catchment scale. However, meeting the WFD objectives should not only depend on a voluntary scheme targeting farmers. In this sense, the problems to obtain data or records about fertiliser inputs are not supposed to occur in farms included in NVZ, as they are subject to cross compliance under the Nitrate Pollution Prevention Regulations 2008 (Statutory Instrument 2008/2349). The requirement, in NVZ, of making a nutrient plan based on soil analysis could have provided, for example, detailed information about the amount of manure applied to soil. As stated above, fertiliser inputs data is crucial to identify CSA using the P Index assessment.
Moreover, the efficacy of the ECSFDI to reduce the risk of P loss to watercourses is difficult to assess. Continuous long term excess P inputs and hence, P accumulation is not a recent episode in an area that has a long tradition of livestock farming. In fact, all the soil samples taken in this study have moderate (26-45 mg kg-1) to high (>45 mg kg-1) Olsen extractable soil P contents. These figures show large P surpluses (>index 2) according to ADAS. Furthermore, these P surpluses determine the soil retention of P inputs due to a lower sorption capacity [63]. This is associated with the risk of P leaching due to increasing amounts of P into the labile pool [64].
In spite of the lack of some data linked to fertiliser inputs, the risk maps showed a higher risk of P loss for some farms during the PIV_W period. This is strongly associated with the application rate of manure to soil in this time of year [49]. The role played by regulations is determinant in these results and in turn, makes difficult to understand the current policies. The obligation of meeting good status of waters by 2015 becomes a hard task when there are farms that can even be exempt from meeting the SSAFO requirements. As stated above, 90 of the slurry store systems were constructed before 1991. Furthermore, the location of these farms in a non NVZ exempts them from providing storage for organic manures for at least 5 months. This rule aims at preventing the spread of manure in the winter period and agrees with studies that have demonstrated a decreased level of P in winter due to larger capacities to store slurry [65]. In addition to this, the existing limitations of these farms to store slurry pose even a higher risk of nutrients loss to watercourses due to the effects of local conditions. In this sense, the humid weather in the study area, bring about a substantial number of slurry applications under wet conditions according to the farmers. These farms practices are considered in the risk model. The P Index assessment reflects different risk values depending on the timing of spreading manure and mineral fertilisers. Higher values are assigned to manure applications in winter, as this is associated with factors such as reduced soil permeability during freezing due to ice formation [66].
Moreover, despite no mineral P was applied in any of the farm fields in winter, P Index assessment assigns the same weight to manure and fertiliser application in the winter period. However, Wood [67] reported higher concentrations of Total P loss in surface runoff for winter application of manure in comparison with results obtained for soils where only P mineral fertiliser had been applied. This is associated with higher risk of losing Soluble Reactive Phosphorus (SRP) through leaching when manure is applied in winter months [68].
Spreading techniques
The risk model applied in this study takes into account not only the time of year but also the method used to apply fertiliser. Nevertheless, it should have been desirable to find not only one farm using a technique similar to the shallow-injection method in order to obtain different results of the impact that these methods can cause on the risk maps. Despite this limitation, figures for Farm 5 obtained from the risk maps (Figure 5) showed lower levels of risk associated with the use of shallowinjection method in comparison with splash plate methods. This is linked to larger amounts of P absorbed by the upper soil layers due to the direct contact of slurry with the soil [50,69]. Thus, this method contributes to decreasing the SRP loss to freshwaters [69].
Regarding the time of year when different techniques such as splash plate or swallow injection are used, deserves special attention. Although this model represents the risk areas of P loss to watercourses, this does not mean that it shows the risk of ecological impact on the streams network. In this sense, it should be considered that the SRP loss occurred in winter months does not cause the same effect on rivers than in spring/summer months due to different rates of biological activity [10,62]. Therefore, the use of the shallow-injection method in spring/summer substantially reduces the risk of waters eutrophication.
Moreover, there is some strong evidence for suggesting that financial pressures are more important than environmental demands. In this regard, it is hard to persuade farmers to use more effective techniques when this involves an important cost [51] and they are not obliged by regulations. In fact, farmers assured to use splash plate methods as the alternatives are more expensive.
Source characteristics
The P Index assessment considers the most important factors associated with diffuse agricultural P pollution [27] however, existing P sources not included in the group of diffuse sources may represent a significant problem when P loss from livestock farms is assessed. During the farm visits, it was observed that some of the farms posed a high risk of P loss due to runoff coming from hardstandings that receive fresh faecal material very frequently (Figure 8). This runoff, highly contaminated in nutrients [70] is the result of management operations, in particular, movement of livestock wastes and rainfall events. Also important is the rise of the proportion of P in soluble form transported in this type of run-off. The presence of tracks, and overflows coming from these hardstandings result in shorter interactions between subsoil and runoff and hence, larger amounts of SRP reaching freshwaters [49]. In addition to this, it should be stressed that this type of management operations occur throughout the year and hence, SRP delivered to surface waters in spring/summer periods contributes to increasing the risk of rivers eutrophication [10].
Transport management
All the soil samples taken from the study farms had pH values considered acidic (Figure 7b). Regarding this, P sorption and release processes in these acidic soils are greatly influenced by clay content which in turn, links to P losses by leaching [15]. In this study, soil texture alongside soil organic matter which is another sink of P [15], were taken into account in order to estimate the resistance of soil to be detached and transport by rainfall and runoff [71]. This is helpful to estimate the risk of P loss associated with particulate material (PP). In fact, Walling [72] reported important relative contributions to the total PP flux coming from grassland areas in UK catchments.
However, the modified Danish P Index adds another transport factor in order to assess the leaching potential based on the type of soil [73]. According to Andersen [73] the soils assessed in this study should be assigned the highest risk of P leaching due to its low P binding capacity (organic soils). Nonetheless, the P Index implemented in this study first requires to be examined according to local conditions in order to introduce any possible modification.
Another relevant aspect associated with soil erosion is derived from cattle trampling stream banks. This type of event is likely to occur in all those farms that were reported to have not fenced off its waterways and when the rates of biological activity are at its highest. This means that the cattle housing period coincides with the water eutrophication risk at its lowest (winter months).
The implemented risk model is based on the RUSLE which in turn, was developed to predict sheet and rill erosion. However, this does not include the effects of concentrated runoff or soil erosion events derived from animal trampling [74]. The clearest example of this type of event was reported to occur in Farm 1 (Figure 9).
In this sense, the risk map obtained for Farm 1 (Figure 10) when the ecological risk is at its highest, lacks of including the risk of PP derived from existing stream bank erosion.
Furthermore, it seems that the importance of the effects of PP on rivers ecosystems changes in spatial and temporal scales, as the key factor is the bioavailability of P for algal proliferation. Jarvie [10] highlighted the small relative contribution of PP derived from agriculture to water eutrophication in lowland rivers in comparison with sewage effluent sources. Therefore SRP desorption from sediment particles represents an ecological risk in individual reaches of rivers where SRP concentrations are low at times of eutrophication risk [4,10,75].
Moreover, transport factors linked to sources not included in the group of diffuse sources were found in Farm 4 (Figure 11). In this regard, steep slope and short distance of these intermediate sources to watercourses significantly increase the risk of nutrients loss [33]. Furthermore, it should be pointed out that Farm 4 received in 2009 a report from the ECSFDI where several recommendations were made in order to prevent polluted water from entering the nearby stream.
However, no improvements have been undertaken. This farm can be considered as an example that cast doubt on the current policies that aim at reducing water pollution at the catchment scale.
This study has yielded that there is significantly more room for improving the management in livestock farms located in non NVZs. Most of the farms apply manure without taking into account the nutrient content of manure as well as the soil nutrient pools. The manure application rate would clearly exceed the restrictions in NVZs, as farmers are not allowed to spread organic fertilisers in NVZs during winter months due to the higher risk of nutrients loss to freshwaters.
In the case of the study farms, the limited store capacity determines the high frequency of manure applications, as most the farmers would be willing to reduce these application rates. Furthermore, the humid weather conditions along with the methods used by farmers to spread manure are factors that exert a strong influence on the risk of P loss in the study area. Nevertheless, the risk pose by rainfall events can be improve in the implemented risk model as long as rainfall intensity data are available. It should be stressed that large amounts of nutrients are flushed out from lands during a few heavy rainfall events [76]. This is particularly relevant when manure is applied and left exposed on the surface (splash-plate method).
Although this study has not demonstrated a significant relationship between P application rates and P Index, it should be pointed out that P surpluses found in the soil are not surprising according to the nutrient management undertaken in most of the study farms. Nevertheless the lack of accurate data for manure application rates could have been decisive for the statistical results. Furthermore this links to the lack of conditions such as current Cross-Compliance rules that apply to NVZs. All these factors contribute to P accumulation in soil and hence, increasing the risk of P loss to freshwaters.
Moreover, the risk maps obtained in this study are the first step to identify the areas where resources should focus in order to achieve the WFD objectives. In fact, the two risk maps obtained for each farm (PIV_S&PIV_W) can provide more accurate information about the risk of P loss in winter months as well as the problematic areas when the ecological risk is higher (spring/summer period).
However, the P index assessment used in this study should be tested in order to examine its capability to rank measured annual diffuse total P losses from the Upper Wensleydale catchment. The results obtained should be analysed in order to introduce possible modifications in the P Index, according to local conditions such as major P loss pathways in the catchment area.
Regarding the current policies that aim at reducing nutrients loadings in freshwaters, we could say that financial pressures on farmers are the major obstacle to implement most the measures recommended by the ECSFDI. In this sense, it would be desirable to increase the financial rewards and incentives if we take into account the current drop in milk prices, according to the farmers. This could help to implement mitigation measures such as increase the slurry storage capacity, and reduce the number of intermediate sources of nutrients. Furthermore, these measures should be accompanied by environmental requirements, as voluntary schemes are not resulting in substantial improvements in livestock management. Finally, it could be necessary to include an increase threat of prosecution.
The author thanks Yorkshire Dales National Park Authority for access to data from the study area. Jamie Duncan from Environment Agency who helped by providing meteorological data. I am also grateful to David Higgins from Yorkshire Dales Rivers Trust as this research had never been accomplished without his collaboration and cooperation. Lastly, I offer my regards to all lecturers and supervisor for their unlimited support during the study period.