Journal of Fertilization: In Vitro - IVF-Worldwide, Reproductive Medicine, Genetics & Stem Cell Biol
Open Access
ISSN: 2375-4508
ISSN: 2375-4508
Review - (2021)Volume 9, Issue 4
The use of extracellular or circulating nucleic acids (cfDNA), as a diagnostic or prognostic tool in oncology, has been widely documented. However, their use in gynecology and obstetrics as non-invasive biomarkers in the management of infertility has become recurrent. Circulating nucleic acids are made up of: free DNA which can be a long or short strand of DNA resulting from apoptotic or necrotic processes, free RNA containing it: micro-RNAs (miRNA) which are ribonucleic acids (RNA) short single-stranded capable of inhibiting the production of proteins from a gene, Piwi interacting RNAs (piRNAs) which are small RNAs expressed in germ cells or even in early embryos, and small RNAs interfering (siRNA) which are small RNAs capable of binding specifically to a messenger RNA sequence and of preventing gene expression by cleaving that RNA. The presence of circulating nucleic acids in many biological fluids such as urine, seminal plasma and serum, the fact that they are easy to detect, the variation in their level depending on the pathophysiological conditions of the body and their involvement in many biological processes such as folliculogenesis, steroidogenesis and spermatogenesis make circulating nucleic acids important and interesting biomarkers for the management of male infertility. They constitute a real complementary aid for the practitioners of medically assisted procreation. Therefore, circulating nucleic acids are a promising avenue in the prevention of implantation failures. In this article we will seek to further assert their importance in the management of male infertility, highlighting their different uses.
Cell-free DNA, Necrotic, Piwi interacting, Folliculogenesis and biomarkers
The use of circulating or free nucleic acids as potential biomarkers in the management of human infertility, particularly in obstetric gynecology, is no longer to be discussed. They constitute of diagnostic and/or prognostic tools of choice in human pathology because they are easily detectable in biological fluids [1], their level varies according to the physiopathological conditions of the body and finally their involvement in many biological processes steroidogenesis permatogenesis. They are commonly used in the management of female infertility as non-invasive biomarkers in the detection and/or monitoring of pathologies of pregnancy, fetal and/or embryonic anomalies and finally in the evaluation. The functional state of the ovary. In this literature review, we will explore the different components of circulating nucleic acids and try to provide the necessary scientific data to justify their interest and their possible applications in PMA.
Free nucleic acids constitute a new source of diagnostic and/or prognostic biomarkers in human pathology. Recent data suggests that they play a vital role in the management of male infertility. Free nucleic acids are free DNA (cfDNA ) (They are molecules present in the form of short (70 to 200 base pairs) or long fragments (up to 21kb) in the body) [2], and free RNA (cfRNA) which consists of nucleotides of three large non-coding RNAs: Piwi interacting RNA (piRNA) (of the order of 24 to 31 nucleotides and having the function of blocking the activity of mobile elements present in DNA), micro-RNA (miRNA) (short strands of non-coding RNA of the order of 19 to 25 nucleotides and whose main role is to block the translation of proteins in the mRNA to which they are attached) and Small interfering RNA (siRNA) (they interfere with RNA in the same way as microRNAs and capable of specifically bind to an mRNA sequence) (Figure 1) [3,4].
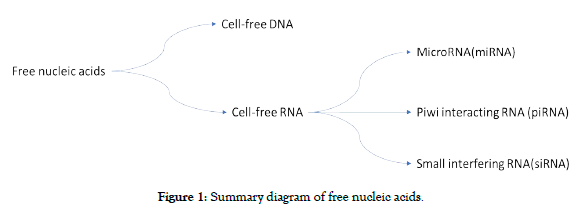
Figure 1. Summary diagram of free nucleic acids.
Even if at present, very few studies have focused on the determination of free nucleic acids in female fertility, it has been shown that there is an increase in the levels of free DNA and/ or microRNAs circulating in the body. Particularly in the plasma and follicular fluids of patients with implantation failures [5-8]. These variations in serum levels could be related to increased cell lysis that is not recognized etiologically. The level of cell-free DNA varies according to the pathophysiological conditions of the organism [9]. However, there appears to be a close relationship between cell-free nucleic acid levels in follicular fluids, maternal plasma, but also embryonic culture media and embryo quality [10]. Thus, the choice of embryos would not only depend on the simple morphological aspect, but also on its environment, which is more or less rich in acellular DNA. Several studies have shown that the expression of microRNAs varies in certain pathologies responsible for female infertility, such as endometriosis [11,12], micropolycystic ovary syndrome (POCS) [13] and premature ovarian failure (POF) [14]. Indeed, Wang et al. reported increased expression of miR-199a and miR-222 and decreased expression of miR-145 and miR-542-3p in patients with endometriosis [15]. In addition, Murri et al, demonstrated that the expression of miR- 21, miR-27b, miR-103 and miR-155 is higher in SOMPK [16]. According to Yang et al, increased expression of miR-146a, miR- 23a, miR-27a and miR-126 and decreased expression of let-7c and miR-144 are observed in the serum of patients with’ POF [17] (Figure 2). This likely impact on fertility could radically alter the practice of reproductive biology. Indeed, one of the major problems of medically assisted reproduction is the low rate of implantation and pregnancy of some patients. It is only in the last fifty years that the existence of non-coding cell-free DNA fragments has been demonstrated in the different biological fluids of the organism. Thus, the determination of these acellular DNA fragments would allow non-invasive prediction of endometrial and oocyte qualities and indirectly embryonic qualities [18-21] of certain patients with implantation failures.
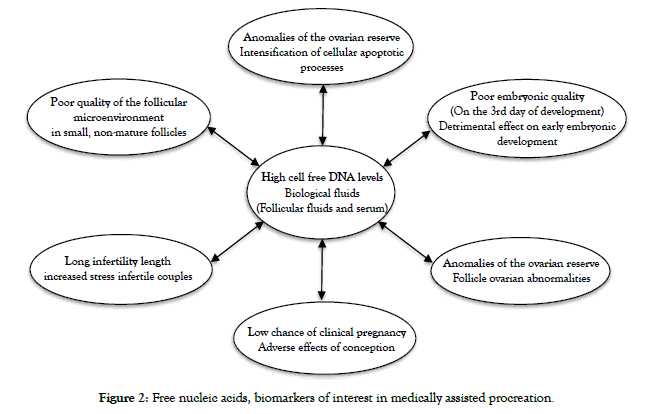
Figure 2. Free nucleic acids, biomarkers of interest in medically assisted procreation.
In addition to the female side and implantation, the exploration of male fertility has made many advances. Cell-free DNA is detectable in human semen. Its concentration in semen is much higher than in other body fluids [22]. Its presence and concentration are directly correlated with semen parameters such as velocity, morphology and motility [23]. According to Li et al, the level of cfDNA is higher in the seminal plasma of patients with defective sperm parameters [24]. These observations explain the use of cfDNA in the search for biological markers of sperm quality. Steger's team detected elevated levels of Prm1 and Prm2 and mRNA in semen obtained from patients with failed in vitro fertilization (IVF) compared to those with successful in vitro fertilization [25]. Bansal et al, also found a probable correlation between sperm DNA profile and male infertility [26]. The expression of microRNAs present in semen can be a very interesting approach and a valuable aid in the current practice of semen quality prediction. According to the team of Salas-Huetos et al, human semen contains a stable population of microRNAs related to embryogenesis and spermatogenesis [27]. However, Salas-Huetos et al, showed that miR-34-P, miR-132-3P, mir30C-5P and miR-375 play a role in cell cycle progression and sperm differentiation [27]. In human spermatozoa, piRNAs account for 11% of cfRNAs [28]. PiRNAs are used in the assessment of sperm quality and offer new perspectives for diagnosis, prognosis and treatment in the management of male infertility [29]. In a small cohort, Li et al, showed that seminal acellular DNA levels were significantly higher in azoospermic patients than in patients without sperm abnormalities [30].
Information on Free DNA Detection (CfDNAs) Genetics and Epigenetics
Semen is a mixture of fluids resulting from various secretions from both testes, epididymis, seminal vesicles, Cowper's glands and prostate [30]. The epigenetic information contained in seminal DNA plays an important role in spermatogenesis. It is involved in the reorganization and condensation of the germ cell genome during maturation. Indeed, it reflects the epigenetic aberrations of the testes [31], the problems of male infertility [32].
Non-Obstructive Azoospermia
Due to lack of adequate stimulation by intrinsic gonadotropins or testicular insufficiency, non-obstructive azoospermia is diagnosed in about 10% of infertile men [33,34]. According to Drabovich's team, non-obstructive azoospermia is diagnosed in about 10% of infertile men [35]. The concentration of CfDNA is much higher in semen than in other human body fluids, with a mean value of 1.34 pg/ml in normozoospermia and 2.56 pg/ml in azoospermia [36]. In a recent analysis of the different mRNA and microRNA profiles of patients with non-obstructive azoospermia (NOA) and patients with obstructive azoospermia compared to normozoospermic controls, the team of Li et al, noted differences in profiles compared to normozoospermic control [30]. At the same time, Wang et al, performed studies in patients with non-obstructive azoospermia (NOA) compared to fertile men, and noted a strong decrease in the expression of seven microRNAs (miR-346-5p, miR-122, Mir 149 ± 5p, miR181a, miR-374b, miR-509, and miR-513a-5p) in seminal plasma of NOA patients compared to control [36] (Table 1). However, in a study conducted by Gunes' team on patients with azoospermia, they found additional expression of miR-34c-5p, miR-122, miR-146b-5p, miR-181a, 374b, miR-509-5p, and 513a-5P that is strongly increased in asthenozoospermia [36] (Table 1). Similarly, Wu's team analyzed testicular tissue from patients with ADD and found a significant increase in the expression of miR-141, miR-429 and miR-7-1-3p in plasma. seminal NOA compared to fertile controls [37].
| Diseases | Sample | MicroRNA | CfDNA | Reference |
|---|---|---|---|---|
| Azoospermia | Sperm | 
Figure 1. Surgical training questionnaire |
 |
[50] |
Figure 1. Surgical training questionnaire miR-346-5p, miR-122,Mir 149 + -5p, miR181a, miR-374b, miR-509 and miR-513a-5p |
 |
[52] [35] |
||
| Asthenozoospermia | sperm |  miR-141, miR-429, miR-141, miR-429,miR-7-1-3p and HSA-miR-27a |
 |
[39] [40] |
| Teratozoospermia | Sperm |  SAH-miR-19b-3p, SAH-miR-19b-3p,hsa-miR-28- 5p, SAH-miR-148B, 106B and mir-5P |
 |
[37] |
| Oligozoospermia | Sperm |  mir-34C-5p, Mir-122, mir-34C-5p, Mir-122,miR-14BB-5P, miR -181A, miR-374b, miR-509-5p, miR-531a-5P, miR-21, miR-22, miR-19b and miR-7bis |
 |
[41] [42] |
Table 1: Summary table of Changes in the level of free DNA and miRNAs in some pathologies linked to sperm quality.
Teratozoospermia, Asthenozoospermia and Oligozoospermia
Male fertility can be affected by abnormalities such as: reduced mobility (asthenozoospermia), abnormal morphology (teratozoospermia), undetectable sperm (azoospermia) or a decrease in sperm count (oligozoospermia).
Teratozoospermia: Teratozoospermia is characterized by the presence of more than 85% morphologically abnormal spermatozoa. In the exploration of useful markers to better compensate for male infertility, the team of Milardi D et al, compared the expression profiles of spermatozoa from men with teratozoospermia and found a decreased expression of SAH-miR-19b-3p, hsa-miR-28- 5p, SAH-miR-148B and 106B-mir- 5P compared to controls (Table 1) [38]. This shows the value of using these miRNAs as biomarkers for the management of male infertility.
Asthenozoospermia: In asthenozoospermic patients, the team of Boissonnas et al, noted an increase in the expression of SAHmiR548c- 5p, SAH-miR548c-5p, and SAH-miR-27a, SAH-mi- 548b -5p -548d-5P compared to normozoospermic controls [39]. Abu-Halima and colleagues noted dysregulation of miR-34b-3p in asthenozoospermic patients compared to normozoospermic patients [40]. Similarly, increased expression of HSA-miR-27a was observed in asthenozoospermic patients (Table 1) [41].
Oligozoospermia: The team of Wang et al, compared the miRNA expression profiles of normal and oligozoospermic patients and observed a significantly lower level of expression of miR-34C-5p, miR-122, miR-14BB-5P, miR-181A, miR-374b, miR-509-5p and miR-531a-5P at the control level compared to oligozoospermic samples [42]. The team of Wu et al, found high expression of miR- 19b and miR-7bis in patients with oligospermia [37]. Assou et al, found a significant increase in the expression level of miR-21 and miR-22. However, finding a cut-off value for the diagnosis and prognosis of male infertility remains a problem (Table 1) [43].
Idiopathic male infertility is defined as the absence of a causal factor in sperm analysis when the sperm has abnormalities such as azoospermia, oligozoospermia, asthenozoospermia or teratozoospermia [44]. It can be affected by other factors such as: DNA fragmentation, oxidative stress, methylation or protein.
DNA fragmentation
The DNA fragmentation index is an important factor in the etiology of male infertility [45] or even a good indicator of the potential of conventional semen parameters [46]. Indeed, spermatozoa with normal parameters may have DNA damage [47]. A study on the etiology of age-related male infertility showed that increased levels of the DNA fragmentation index can lead to reduced sperm motility [48]. Similarly, the team of Twyma-Saint Victor et al, in a published study of three patients with male infertility, found that the proportion of sperm with DNA fragmentation ranged from 4.4 to 28% compared to a DNA fragmentation percentage of 1.20 ± 0.95% in controls [49].
Apart from the DNA fragmentation index, various conditions such as chromatin decondensation index, stress and methylation can affect males [50].
Oxidative stress
Even though a low concentration of reactive oxygen species (ROS) is necessary for the critical steps of fertilization, namely capacitation, acrosome reaction and fusion between oocyte and spermatozoon [51]. It appears that cellular stress in all its forms can lead to an increase in the rate of the DNA fragmentation index and affect male fertility. Moreover, the team of Twigg et al, have shown that seminal reactive oxygen species (ROS) levels can lead to sperm damage resulting in male infertility. However, the lack of consensus on the pathophysiological limits of reactive oxygen species (ROS) remains the crucial problem [52].
Methylation
DNA methylation modifies the genetic material. It is a causal factor in infertility [53]. Increased levels of cyclic adenosine monophosphate (cAMP) are a negative factor for normal sperm motility and morphology. The teams of Poongotha and al, have shown that methylation contributes to the increase in Mesodermal Specific Transcriptase (MEST) in association with abnormal sperm parameters and male infertility [54].
Proteomics
In seminal fluid, sperm accounts for 10% of the total volume of ejaculation while 90% is a diverse molecular composition. The specific protein concentration provides a rich source of potential biomarkers in the assessment of male fertility [53]. The team of Mitsumoto observed in infertile men a decrease in the protein (DJ- 1-A) involved in the regulation of oxidative stress [55]. Diamandis also found a positive correlation between the seminal concentration of Prostaglandin-D-Synthase (PTGDS) with the mobility and normal morphology of spermatozoa [56]. The proteomic study conducted in the search for biological markers of azoospermia conducted by Bieniek showed that proteins such as, PTGDS, ACRV1, LGALS3BP, ECM1 and TEX101 are seminal biomarkers for the evaluation of male infertility [57].
The development of biomarkers for the diagnosis of male infertility, the provision of assistance for drug development and its application at the human level cannot be possible without having gone through the animal model, including the mouse [58]. The experts in reproductive biology use several animal models. But in this part, we will only talk about the proteomic technology of sperm. The mouse model led to the identification of 52 proteins at the spermatic level [59]. Bleil et al., from the sperm of the boar have identified the surface proteins of the spermatozoa responsible for connecting the spermatozoon to the oocyte [60]. The list of animals used as models is very long. The dog remains the best experimental model for comparative studies in humans because of the similarity of the prostate [61]. The main challenge according to Naughton is the translation of knowledge acquired from its animal models to the male infertility clinic for an improvement of existing treatment, the development of an accurate diagnosis and the formulation of male contraceptives with minimal side effects [62].
The use of nucleic acids as biomarkers of male fertility remains an innovative approach and is extremely promising because it offers new perspectives from a diagnostic as well as prognostic points of view. This is because of the relationship between the level of CfDNA and the presence or absence of spermatozoa. In addition, it is a noninvasive procedure and therefore reduces the risks the patient is exposed to.
Authors have declared that no competing interests exist.
Citation: Mbaye MM, El Khalfi B, Zakaria A, Louanjli N, Zakaria M, Soukri A (2021) The Interest of Circulating DNA in the Management of Infertility, Especially in Men. J Fertil In vitro IVF Worldw Reprod Med Genet Stem Cell Biol 9:4. doi: 10.35248/2375-4508.21.9.239.
Received: 27-Apr-2021 Accepted: 03-Jun-2021 Published: 10-Jun-2021 , DOI: 10.35248/2375-4508.21.9.239
Copyright: © 2021 Mbaye MM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.